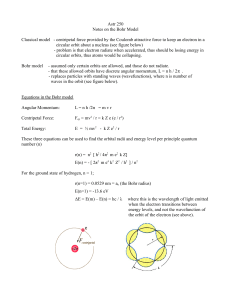
Astr 250 Notes on the Bohr Model Classical model
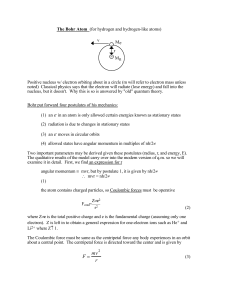
... Astr 250 Notes on the Bohr Model Classical model - centripetal force provided by the Coulomb attractive force to keep an electron in a circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms wo ...
... Astr 250 Notes on the Bohr Model Classical model - centripetal force provided by the Coulomb attractive force to keep an electron in a circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms wo ...
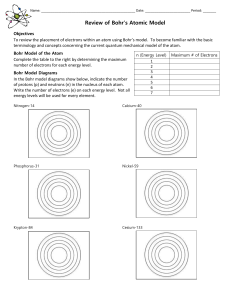
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... Review of Bohr’s Atomic Model Objectives ...
... Review of Bohr’s Atomic Model Objectives ...
F = mv r
... There is one more detail - we have been working with the assumption that we know ni & n f and want to obtain ∆E or λ - we could also perform an experiment that measures λ. Using Planck's law ∆E = hc λ we could obtain ∆E ( and ni, if nf is already known). All of the quantum theory we have done is app ...
... There is one more detail - we have been working with the assumption that we know ni & n f and want to obtain ∆E or λ - we could also perform an experiment that measures λ. Using Planck's law ∆E = hc λ we could obtain ∆E ( and ni, if nf is already known). All of the quantum theory we have done is app ...
lect10
... that tells us that the world, at the quantum level, is governed by statistical law. It rules out “classical” or “naïve” realist views of nature. As an example, consider the following applet demonstrating the Hydrogen atom. ...
... that tells us that the world, at the quantum level, is governed by statistical law. It rules out “classical” or “naïve” realist views of nature. As an example, consider the following applet demonstrating the Hydrogen atom. ...
المحاضرة الثانية اساسيات الكم
... An increase in the principal quantum number from n = 1 to n=∞ has a special significance; it corresponds to the ionization of the atom and the ionization energy, IE, can be determined as shown in the following example. Values of IEs are quoted per mole of atoms: ...
... An increase in the principal quantum number from n = 1 to n=∞ has a special significance; it corresponds to the ionization of the atom and the ionization energy, IE, can be determined as shown in the following example. Values of IEs are quoted per mole of atoms: ...
Bohr`s atomic model
... Bohr’s model cannot explain everything about atoms and line spectra and it has been superseded by more sophisticated models, but it was a major advance in atomic theory and quantum mechanics. In 1922 Bohr received the Nobel prize for physics. In a long working life at Copenhagen University he develo ...
... Bohr’s model cannot explain everything about atoms and line spectra and it has been superseded by more sophisticated models, but it was a major advance in atomic theory and quantum mechanics. In 1922 Bohr received the Nobel prize for physics. In a long working life at Copenhagen University he develo ...
Slide 1
... The end of classical physics: photons, electrons, atoms Last time: Discovery of the electron: Charge quantization photo-electric effect: light behaves as a particle Discovery of the nucleus: Rutherford scattering experiment Today: Atomic spectra: emission and absorption. Evidence for atomic ener ...
... The end of classical physics: photons, electrons, atoms Last time: Discovery of the electron: Charge quantization photo-electric effect: light behaves as a particle Discovery of the nucleus: Rutherford scattering experiment Today: Atomic spectra: emission and absorption. Evidence for atomic ener ...
Chap 2 Solns
... electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (b) Two important refinements resulting from the wave-mechanical atomic model are (1) that electron position is described in terms of a probability distribution, and (2) electron energy is quantize ...
... electrons are particles moving in discrete orbitals, and (2) electron energy is quantized into shells. (b) Two important refinements resulting from the wave-mechanical atomic model are (1) that electron position is described in terms of a probability distribution, and (2) electron energy is quantize ...
Electron Orbits
... "It might interest you to know that when we made the experiments that we did not know Bohr's theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other ...
... "It might interest you to know that when we made the experiments that we did not know Bohr's theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other ...
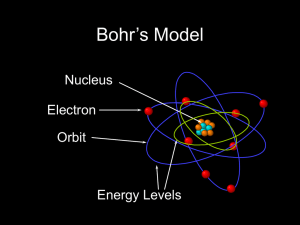
Rutherford–Bohr model
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
... atomic nucleus and where an electron jump between orbits is accompanied by an emitted or absorbed amount of electromagnetic energy (hν).[1] The orbits in which the electron may travel are shown as grey circles; their radius increases as n2, where n is the principal quantum number. The 3 → 2 transiti ...
Laboratory 3: Determining the Critical Potentials for Helium: The
... electrons though mercury vapor and observing the loss of kinetic energy when an electron struck a mercury atom and excited it from its lowest energy state to a higher one. This occurred at 4.9 eV and all electrons with at least this amount of energy would lose only 4.9 eV showing the quantum nature ...
... electrons though mercury vapor and observing the loss of kinetic energy when an electron struck a mercury atom and excited it from its lowest energy state to a higher one. This occurred at 4.9 eV and all electrons with at least this amount of energy would lose only 4.9 eV showing the quantum nature ...
Franck–Hertz Experiment www.AssignmentPoint.com The Franck
... quantum nature of atoms, and thus "transformed our understanding of the world". It was presented on April 24, 1914 to the German Physical Society in a paper by James Franck and Gustav Hertz. Franck and Hertz had designed a vacuum tube for studying energetic electrons that flew through a thin vapor o ...
... quantum nature of atoms, and thus "transformed our understanding of the world". It was presented on April 24, 1914 to the German Physical Society in a paper by James Franck and Gustav Hertz. Franck and Hertz had designed a vacuum tube for studying energetic electrons that flew through a thin vapor o ...
James Franck

James Franck (26 August 1882 – 21 May 1964) was a German physicist who won the 1925 Nobel Prize for Physics with Gustav Hertz ""for their discovery of the laws governing the impact of an electron upon an atom"". He completed his doctorate in 1906 and his habilitation in 1911 at the Frederick William University in Berlin, where he lectured and taught until 1918, having reached the position of professor extraordinarius. He served as a volunteer in the German Army during World War I. He was seriously injured in 1917 in a gas attack and was awarded the Iron Cross 1st Class.Franck became the Head of the Physics Division of the Kaiser Wilhelm Gesellschaft for Physical Chemistry. In 1920, Franck became professor ordinarius of experimental physics and Director of the Second Institute for Experimental Physics at the University of Göttingen. While there he worked on quantum physics with Max Born, who was Director of the Institute of Theoretical Physics. His work included the Franck–Hertz experiment, an important confirmation of the Bohr model of the atom. He promoted the careers of women in physics, notably Lise Meitner, Hertha Sponer and Hilde Levi.After the NSDAP came to power in Germany in 1933, Franck resigned his post in protest against the dismissal of fellow academics. He assisted Frederick Lindemann in helping dismissed Jewish scientists find work overseas, before he left Germany in November 1933. After a year at the Niels Bohr Institute in Denmark, he moved to the United States, where he worked at Johns Hopkins University in Baltimore and then the University of Chicago. During this period he became interested in photosynthesis.Franck participated in the Manhattan Project during World War II as Director of the Chemistry Division of the Metallurgical Laboratory. He was also the chairman of the Committee on Political and Social Problems regarding the atomic bomb, which is best known for the compilation of the Franck Report, which recommended that the atomic bombs not be used on the Japanese cities without warning.