Periodic Table Worksheet
... 4. Elements of Group 3-12 are called: Transition Elements *5. Group 17 elements are called: Halogens (charge: -1) *6. Group 18 elements are called: Noble Gases 7. An element with both metallic and non metallic properties is called a: metalloid or semi-metal 8. The majority of elements in the periodi ...
... 4. Elements of Group 3-12 are called: Transition Elements *5. Group 17 elements are called: Halogens (charge: -1) *6. Group 18 elements are called: Noble Gases 7. An element with both metallic and non metallic properties is called a: metalloid or semi-metal 8. The majority of elements in the periodi ...
Periodic Table Worksheet
... 4. Elements of Group 3-12 are called: Transition Elements *5. Group 17 elements are called: Halogens (charge: -1) *6. Group 18 elements are called: Noble Gases 7. An element with both metallic and non metallic properties is called a: metalloid or semi-metal 8. The majority of elements in the periodi ...
... 4. Elements of Group 3-12 are called: Transition Elements *5. Group 17 elements are called: Halogens (charge: -1) *6. Group 18 elements are called: Noble Gases 7. An element with both metallic and non metallic properties is called a: metalloid or semi-metal 8. The majority of elements in the periodi ...
ionic compound - East Penn School District
... Many are gases at room temperature Lack luster Low melting points ...
... Many are gases at room temperature Lack luster Low melting points ...
8th Science LF Sept 7-11
... Investigating the Periodic Table Video on Discovery Education Periodic Table Song and Meet the Elements Song Atom Model - Bellringer Metals, nonmetals, and metalloids foldable Periodic Table Brain Pop Video Quiz Color the periodic table to distinguish location of metals, non-metals, and metalloids. ...
... Investigating the Periodic Table Video on Discovery Education Periodic Table Song and Meet the Elements Song Atom Model - Bellringer Metals, nonmetals, and metalloids foldable Periodic Table Brain Pop Video Quiz Color the periodic table to distinguish location of metals, non-metals, and metalloids. ...
Atoms - Schoolwires.net
... • Isotopic Abundance is the percent or fraction of each isotope found in nature. ...
... • Isotopic Abundance is the percent or fraction of each isotope found in nature. ...
C6_rev - boswellsrcd
... flavouring or vitamin, drugs etc. They are made to high levels of purity. Usually in laboratories. • bulk chemicals A chemical product that is made in large amounts, very cheaply and often used to make other chemicals or to process other materials e.g. bleach, solvents, sulphuric acid etc. Usually m ...
... flavouring or vitamin, drugs etc. They are made to high levels of purity. Usually in laboratories. • bulk chemicals A chemical product that is made in large amounts, very cheaply and often used to make other chemicals or to process other materials e.g. bleach, solvents, sulphuric acid etc. Usually m ...
Ch 5 Notes
... • Hydrogen is in group 1 but is not an alkali metal, because it is only 1 proton and 1 electron (no neutrons) • Its properties are closer to a nonmetals than to a metal • it is a colorless, odorless, explosive gas with oxygen ...
... • Hydrogen is in group 1 but is not an alkali metal, because it is only 1 proton and 1 electron (no neutrons) • Its properties are closer to a nonmetals than to a metal • it is a colorless, odorless, explosive gas with oxygen ...
d. Group 1
... An element with the smallest anionic (negativeionic) radius would be found on the periodic table in a. Group 1, Period 7. b. Group 3, Period 4. c. Group 5, Period 3. d. Group 17, Period 2. ...
... An element with the smallest anionic (negativeionic) radius would be found on the periodic table in a. Group 1, Period 7. b. Group 3, Period 4. c. Group 5, Period 3. d. Group 17, Period 2. ...
Ch. 14 notes (teacher)3
... tend to __________ e-’s anyway, and this makes them highly ________________ attracted to e-’s when forming a chemical bond. ...
... tend to __________ e-’s anyway, and this makes them highly ________________ attracted to e-’s when forming a chemical bond. ...
Name
... 11. Elements of Group 1 are called ____________________________ 12. Elements of Group 2 are called ____________________________ 13. Elements of Group 3-11 are called __________________________________ 14. As you go from left to right across the periodic table, the elements go from ( metals / nonmeta ...
... 11. Elements of Group 1 are called ____________________________ 12. Elements of Group 2 are called ____________________________ 13. Elements of Group 3-11 are called __________________________________ 14. As you go from left to right across the periodic table, the elements go from ( metals / nonmeta ...
Lesson 7.8 Basic Properties of the Main Group Elements Suggested
... stored under water. In air it bursts into flame reacting with O2 to give the acidic oxides P4O6 and P4O10. Other allotropes, red and black phosphorus are much less reactive. Grey arsenic, the common form of the element, is a brittle solid with metallic luster. Yellow arsenic is an unstable, crystall ...
... stored under water. In air it bursts into flame reacting with O2 to give the acidic oxides P4O6 and P4O10. Other allotropes, red and black phosphorus are much less reactive. Grey arsenic, the common form of the element, is a brittle solid with metallic luster. Yellow arsenic is an unstable, crystall ...
AP Chemistry Summer Assignment
... 31.An extensive property is one that depends on the amount of the sample. Which of the following properties are extensive? a. volume b. density c. temperature d. energy e. melting point. F. pressure 32.A hydrated compound has an analysis of 18.29% Ca, 32.37% Cl, and 49.34% water. What is its formula ...
... 31.An extensive property is one that depends on the amount of the sample. Which of the following properties are extensive? a. volume b. density c. temperature d. energy e. melting point. F. pressure 32.A hydrated compound has an analysis of 18.29% Ca, 32.37% Cl, and 49.34% water. What is its formula ...
periodic-data-and-trends-assign-2016
... Because the repulsion forces are a lot stronger and hold the atoms in closer to the protons. ...
... Because the repulsion forces are a lot stronger and hold the atoms in closer to the protons. ...
Chapter6
... Properties in a period vary from left to right, but this pattern of varying properties repeats from one period to the next. Periodic Law - When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Metals, Nonmetals, and ...
... Properties in a period vary from left to right, but this pattern of varying properties repeats from one period to the next. Periodic Law - When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties. Metals, Nonmetals, and ...
Intermediate 1 Chemistry - Deans Community High School
... 1) All of the noble gases have a full outer shell, so they are very _____________ 2) They all have low melting and boiling points 3) Helium is lighter then air and is used in balloons and airships (as well as for talking in a silly voice) 4) Argon is used in light bulbs (because it is so unreactive) ...
... 1) All of the noble gases have a full outer shell, so they are very _____________ 2) They all have low melting and boiling points 3) Helium is lighter then air and is used in balloons and airships (as well as for talking in a silly voice) 4) Argon is used in light bulbs (because it is so unreactive) ...
Formula and The Mole
... Z = bromine solution b) Cracking is breaking up a large molecule (hydrocarbon) into a mixture of smaller and more useful molecules. c) To allow the reaction to take place at a lower ...
... Z = bromine solution b) Cracking is breaking up a large molecule (hydrocarbon) into a mixture of smaller and more useful molecules. c) To allow the reaction to take place at a lower ...
The Periodic Table
... • The elements are listed on the Periodic Table in FROM LEAST TO GREATEST, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. • The element’s ATOMIC NUMBER is based on the number of protons in each atom of that elem ...
... • The elements are listed on the Periodic Table in FROM LEAST TO GREATEST, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. • The element’s ATOMIC NUMBER is based on the number of protons in each atom of that elem ...
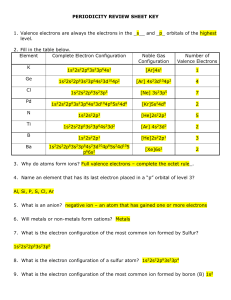
periodicity review sheet key
... Using the graphs from your notes, period table or text book, answer the following with: increases(I), decreases (D), or remains the same (R). __R___ 14. As the number of neutrons in an atom of a given element increases, its atomic number generally ________________ . __I___ 15. Going down a group of ...
... Using the graphs from your notes, period table or text book, answer the following with: increases(I), decreases (D), or remains the same (R). __R___ 14. As the number of neutrons in an atom of a given element increases, its atomic number generally ________________ . __I___ 15. Going down a group of ...
word - My eCoach
... ____ 27. In which of the following is the name and formula given correctly? a. sodium oxide, NaO c. copper(II) chloride, CuCl2 b. barium nitride, Ba(NO3)2 d. iron(II) oxide, Fe2O3 ____ 28. An element with an electronegativity of 0.9 bonds with an element with an electronegativity of 3.1. Which of th ...
... ____ 27. In which of the following is the name and formula given correctly? a. sodium oxide, NaO c. copper(II) chloride, CuCl2 b. barium nitride, Ba(NO3)2 d. iron(II) oxide, Fe2O3 ____ 28. An element with an electronegativity of 0.9 bonds with an element with an electronegativity of 3.1. Which of th ...
What makes a group of elements
... elements known at the time. Mendeleev wrote the symbol for each element on a card along with its relative atomic mass. Arranged elements in order of increasing atomic mass. He is considered the “father of the ...
... elements known at the time. Mendeleev wrote the symbol for each element on a card along with its relative atomic mass. Arranged elements in order of increasing atomic mass. He is considered the “father of the ...
Chapter 3 Periodic Table
... • http://www.youtube.com/watch?v=dhEkyYUXSo • http://www.youtube.com/watch?v=x4WEq ...
... • http://www.youtube.com/watch?v=dhEkyYUXSo • http://www.youtube.com/watch?v=x4WEq ...
Periodic table of elements
... Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's ...
... Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's ...
The Periodic Table
... Due to increasing nuclear charge. Noble gases do not lose electrons easily (low reactivity). • IE generally decreases down the groups. ...
... Due to increasing nuclear charge. Noble gases do not lose electrons easily (low reactivity). • IE generally decreases down the groups. ...
Reactivity of Atoms Based on Their Placement in The Periodic Table
... current varies with temperature Example: Silicon (Si) is a poor conductor at room temperature but a good conductor at high ...
... current varies with temperature Example: Silicon (Si) is a poor conductor at room temperature but a good conductor at high ...