Stars and Elements
... • Hydrogen and helium formed in the early moments after the Big Bang. They are the simplest naturally occurring elements and were formed in great quantities as the early Universe cooled. ...
... • Hydrogen and helium formed in the early moments after the Big Bang. They are the simplest naturally occurring elements and were formed in great quantities as the early Universe cooled. ...
Oxidation
... Rules for Assigning Oxidation Numbers 1) The sum of the oxidation numbers will always equal the particle’s charge 2) The oxidation number for a neutral atom is always zero 3) Oxidation numbers for non–VOS metals depend on their group 4) Oxidation numbers for VOS metals are found based on anion 5) O ...
... Rules for Assigning Oxidation Numbers 1) The sum of the oxidation numbers will always equal the particle’s charge 2) The oxidation number for a neutral atom is always zero 3) Oxidation numbers for non–VOS metals depend on their group 4) Oxidation numbers for VOS metals are found based on anion 5) O ...
Oxidation Numbers
... the net charge that an atom would have if the electrons in a covalent bond belonged entirely to the more electronegative atom ...
... the net charge that an atom would have if the electrons in a covalent bond belonged entirely to the more electronegative atom ...
ConcepTest On Simple Redox Reactions
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
... Comment to Instructor: Correct answer is 3. HCl. Since the oxidation number of H is decreasing from +1 to 0, it is undergoing reduction. Zn is being oxidized, and HCl is the “agent” that is causing the Zn to be oxidized. #4 indicates that the student is thinking that the Zn+2in ZnCl2 is undergoing r ...
(+1) + - Edublogs
... For electrons that are shared in these compounds, we assign the shared electrons to the most electronegative element. We are just acting as though the electronegativity difference was large enough for the transfer of electrons to occur. ...
... For electrons that are shared in these compounds, we assign the shared electrons to the most electronegative element. We are just acting as though the electronegativity difference was large enough for the transfer of electrons to occur. ...
Atoms, Ions and Molecules
... properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. 3. Compounds are formed when atoms of two or more elements combine. In a given compound, the relative numbers of at ...
... properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. 3. Compounds are formed when atoms of two or more elements combine. In a given compound, the relative numbers of at ...
Oxidation-Reduction (Redox) Reactions
... can be broken down breaking reaction into half reactions: oxidation (loss of e-): Zn (s) ...
... can be broken down breaking reaction into half reactions: oxidation (loss of e-): Zn (s) ...
- Angelo State University
... • Isotopes of an element have the same # of protons, but different #’s of neutrons. – Isotopes of an element have nearly identical chemical behavior. – A nuclide is the nucleus of an element with a particular combination of protons and neutrons. – A particular nuclide can be indicated by writing the ...
... • Isotopes of an element have the same # of protons, but different #’s of neutrons. – Isotopes of an element have nearly identical chemical behavior. – A nuclide is the nucleus of an element with a particular combination of protons and neutrons. – A particular nuclide can be indicated by writing the ...
Unit 2 Complete 2016 2017
... 6. Define and distinguish between an ion and an atom. 7. Calculate the average atomic mass of an element or isotope percent/relative abundances. 8. Identify the different types of nuclear reactions 9. Compare and contrast fusion and fission. 10. Calculate half-life ...
... 6. Define and distinguish between an ion and an atom. 7. Calculate the average atomic mass of an element or isotope percent/relative abundances. 8. Identify the different types of nuclear reactions 9. Compare and contrast fusion and fission. 10. Calculate half-life ...
Midterm Review Teacher Answer Key December 21, 2011 `see
... Explain, in terms of electron configuration, why atoms of the radioisotope produced by the sixth decay in the U-238 disintegration series do not readily react to form compounds. [1] Use the Periodic Table of the Elements. Radon (Rn, atomic number 86) is a noble gas. It is found in Group 18. Element ...
... Explain, in terms of electron configuration, why atoms of the radioisotope produced by the sixth decay in the U-238 disintegration series do not readily react to form compounds. [1] Use the Periodic Table of the Elements. Radon (Rn, atomic number 86) is a noble gas. It is found in Group 18. Element ...
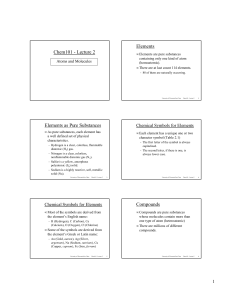
Chem101 - Lecture 2 Elements Elements as Pure
... - Chlorine (Cl) also has two naturally occurring isotopes: one has 18 neutrons and represents 75.78% of all naturally occurring chlorine, the other has 20 neutrons and represents the remaining 24.22% of all naturally occurring chlorine. - Magnesium (Mg) has three naturally occurring isotopes: one ha ...
... - Chlorine (Cl) also has two naturally occurring isotopes: one has 18 neutrons and represents 75.78% of all naturally occurring chlorine, the other has 20 neutrons and represents the remaining 24.22% of all naturally occurring chlorine. - Magnesium (Mg) has three naturally occurring isotopes: one ha ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge. • In other words, effective nuclear charge increases and thus, size decreases. Therefore, the elements of this group have smaller size t ...
... but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge. • In other words, effective nuclear charge increases and thus, size decreases. Therefore, the elements of this group have smaller size t ...
Name: Period:______ Table Number:______
... ___________(Uranium) are naturally occuring elements which can be found to exist somewhere in the earth’s land, water, or air. 57. On the periodic table of elements those elements which have an atomic number of _____ (Neptunium) to ______ (Ununoctium) are synthetic or man made elements which have a ...
... ___________(Uranium) are naturally occuring elements which can be found to exist somewhere in the earth’s land, water, or air. 57. On the periodic table of elements those elements which have an atomic number of _____ (Neptunium) to ______ (Ununoctium) are synthetic or man made elements which have a ...
Isotopes
... all of the atoms of a given element were identical. This idea persisted for over 100 years, until James Chadwick discovered that the nuclei of most atoms contain neutrons as well as protons. (This is a good example of how a theory changes as new observations are made.) After the discovery of the neu ...
... all of the atoms of a given element were identical. This idea persisted for over 100 years, until James Chadwick discovered that the nuclei of most atoms contain neutrons as well as protons. (This is a good example of how a theory changes as new observations are made.) After the discovery of the neu ...
presentation1-elements-atoms-and-isotopes
... Stable and Unstable Isotopes • Some isotopes are stable, while others are radioactive and release particles and energy to decay into a more stable form • Small Nuclei - Atoms which contain up to 20 protons (up to Calcium) are usually stable. • Large Nuclei - Larger nuclei tend to be radioactive. Al ...
... Stable and Unstable Isotopes • Some isotopes are stable, while others are radioactive and release particles and energy to decay into a more stable form • Small Nuclei - Atoms which contain up to 20 protons (up to Calcium) are usually stable. • Large Nuclei - Larger nuclei tend to be radioactive. Al ...
Chapter 2 Atoms and Elements
... weigh a sample of an element, they are weighing a huge number of atoms. The periodic table lists atomic masses of elements in amu per atom, but it would be tremendously helpful if these numbers in the chart could be used when measuring masses in grams. Scientists defined a number of particles (the m ...
... weigh a sample of an element, they are weighing a huge number of atoms. The periodic table lists atomic masses of elements in amu per atom, but it would be tremendously helpful if these numbers in the chart could be used when measuring masses in grams. Scientists defined a number of particles (the m ...
Name: Period:______ Table Number:______
... 51. Each element found on the periodic table of elements has a unique single letter (Hydrogen – H), two letter (Helium – He ) or three letter (Unnilquadiam – Unq) abbreviation which is called the CHEMICAL SYMBOL of that element. P. 83, Bill Nye the Science Guy Video 52. JONS BERZELIUS created the un ...
... 51. Each element found on the periodic table of elements has a unique single letter (Hydrogen – H), two letter (Helium – He ) or three letter (Unnilquadiam – Unq) abbreviation which is called the CHEMICAL SYMBOL of that element. P. 83, Bill Nye the Science Guy Video 52. JONS BERZELIUS created the un ...
Balancing Redox Equations
... Oxidation Number - The charge that an atom would have if the compound in which it were found were ionic. The rules: 1) The sum of the oxidation numbers of the atoms in a molecule must be equal to the overall charge on the molecule. 2) To assign a number to a transition metal ion (not listed in the t ...
... Oxidation Number - The charge that an atom would have if the compound in which it were found were ionic. The rules: 1) The sum of the oxidation numbers of the atoms in a molecule must be equal to the overall charge on the molecule. 2) To assign a number to a transition metal ion (not listed in the t ...
Step 2
... number to each element wherever it appears in the equation. If the reaction is a redox reaction, identify the element that undergoes an increase in oxidation number and the elements the undergoes a decrease. Find the numerical values of the increase and decrease. Determine the smallest whole-number ...
... number to each element wherever it appears in the equation. If the reaction is a redox reaction, identify the element that undergoes an increase in oxidation number and the elements the undergoes a decrease. Find the numerical values of the increase and decrease. Determine the smallest whole-number ...
Competition for Electrons
... track of electrons based on the arbitrary assumption that shared electrons belong to the more electronegative element n Rules for assigning oxidation numbers q Oxidation numbers for atoms that are free elements are always zero q The oxidation numbers of ions are the same as the charge on the ion q S ...
... track of electrons based on the arbitrary assumption that shared electrons belong to the more electronegative element n Rules for assigning oxidation numbers q Oxidation numbers for atoms that are free elements are always zero q The oxidation numbers of ions are the same as the charge on the ion q S ...
- Orangefield ISD
... All atoms of a particular element have the same number of protons and electrons but the number of neutrons in the nucleus can differ. Atoms with the same number of protons but different numbers of neutrons are called isotopes. In nature, most elements are found as mixtures of isotopes. Usually, the ...
... All atoms of a particular element have the same number of protons and electrons but the number of neutrons in the nucleus can differ. Atoms with the same number of protons but different numbers of neutrons are called isotopes. In nature, most elements are found as mixtures of isotopes. Usually, the ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... You have learned that the mass of atoms is measured in atomic mass units. This unit is too small to use in everyday measurement. It would be simpler to have a number of atoms that would have a mass in grams that is equal to the mass of one atom in atomic mass units. The same number would fit all ele ...
... You have learned that the mass of atoms is measured in atomic mass units. This unit is too small to use in everyday measurement. It would be simpler to have a number of atoms that would have a mass in grams that is equal to the mass of one atom in atomic mass units. The same number would fit all ele ...
INTRODUCTION TO CHEMISTRY - Chapter 1
... Calvin Coolidge recognized that chemistry makes significant contributions to our quality of life. From the structural material provided by plastics to medicines, pesticides and combustion, we use the products of chemistry all the time. However, there also sometimes have been accompanying negative co ...
... Calvin Coolidge recognized that chemistry makes significant contributions to our quality of life. From the structural material provided by plastics to medicines, pesticides and combustion, we use the products of chemistry all the time. However, there also sometimes have been accompanying negative co ...
wahideh chemistry eportfolio hw
... Sodium is a member of the alkali metals family. The alkali family consists of elements in Group 1 (IA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Other Group 1 (IA) elements are lithium, potassium, rubidium, cesium, and francium. ...
... Sodium is a member of the alkali metals family. The alkali family consists of elements in Group 1 (IA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to one another. Other Group 1 (IA) elements are lithium, potassium, rubidium, cesium, and francium. ...
The d- and f- Block Element Block Elements The d- and f
... Due to an increase in nuclear charge which accompanies the filling of the inner d orbitals, there is an increase in ionisation enthalpy along each series of the transition elements from left to right. However, many small variations occur. Table 8.2 gives the values for the first three ionisation ent ...
... Due to an increase in nuclear charge which accompanies the filling of the inner d orbitals, there is an increase in ionisation enthalpy along each series of the transition elements from left to right. However, many small variations occur. Table 8.2 gives the values for the first three ionisation ent ...