Chapter 2
... These are cations or anions consisting of groups of atoms that are covalently bonded to each other Examples are NO3-, SO42-, ClO4-, MnO4When more than one appears in a formula unit, the polyatomic ion is put in between parentheses, and a subscript is used to indicate the number of the ions that appe ...
... These are cations or anions consisting of groups of atoms that are covalently bonded to each other Examples are NO3-, SO42-, ClO4-, MnO4When more than one appears in a formula unit, the polyatomic ion is put in between parentheses, and a subscript is used to indicate the number of the ions that appe ...
AGS General Science Chapt 2
... Sometimes scientists can tell what things look like by studying how they act. For example, have you ever seen wind? What does it look like? You might say that wind is leaves blowing or your hair getting messed up. If you say that, you are describing what wind does, not what it looks like. You use th ...
... Sometimes scientists can tell what things look like by studying how they act. For example, have you ever seen wind? What does it look like? You might say that wind is leaves blowing or your hair getting messed up. If you say that, you are describing what wind does, not what it looks like. You use th ...
Chapter 3 Atoms and Elements
... • are better conductors than nonmetals, but not as good as metals • are used as semiconductors and insulators • as a general rule they have the appearance of metals and the properties and characteristics of nonmetals ...
... • are better conductors than nonmetals, but not as good as metals • are used as semiconductors and insulators • as a general rule they have the appearance of metals and the properties and characteristics of nonmetals ...
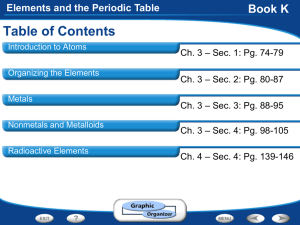
Elements and the Periodic Table
... • The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. ...
... • The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. ...
elements of chemistry unit
... CLASS NOTES REDOX REACTIONS One type of chemical reaction involves the transfer of electrons from one species (species means atoms or groups of atoms) to another. These reactions are called oxidation reduction reactions. REDOX REACTIONS The species that loses electrons is oxidized and the species ga ...
... CLASS NOTES REDOX REACTIONS One type of chemical reaction involves the transfer of electrons from one species (species means atoms or groups of atoms) to another. These reactions are called oxidation reduction reactions. REDOX REACTIONS The species that loses electrons is oxidized and the species ga ...
GCSE Chemistry Textbook sample
... Compounds are difficult to break back down into their elements. Substances in mixtures are not chemically joined to each other. Substances in mixtures can be separated easily by a range of techniques. ...
... Compounds are difficult to break back down into their elements. Substances in mixtures are not chemically joined to each other. Substances in mixtures can be separated easily by a range of techniques. ...
Document
... b) Mixing of Mn(VII) compounds with Mn(IV) compounds to form Mn(VI) compounds in alkaline medium. 2 MnO4- + MnO2 + 4 OH- ...
... b) Mixing of Mn(VII) compounds with Mn(IV) compounds to form Mn(VI) compounds in alkaline medium. 2 MnO4- + MnO2 + 4 OH- ...
Answer - We can offer most test bank and solution manual you need.
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
Answer - TEST BANK 360
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
Module-2-s-and-d-elements - Львівський національний медичний
... physical and chemical properties occur at specific intervals. These groups of elements with similar physical and chemical properties are called families, examples of which are the alkali metals, alkaline earth metals, rare earth elements, halogens, and the noble gases. When two atoms have the same a ...
... physical and chemical properties occur at specific intervals. These groups of elements with similar physical and chemical properties are called families, examples of which are the alkali metals, alkaline earth metals, rare earth elements, halogens, and the noble gases. When two atoms have the same a ...
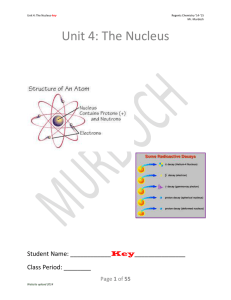
protons, neutrons, electrons
... SC.A.2.3.2.7.3 – determines the mass number and atomic number of an atom from the number of protons and neutrons SC.H.3.3.1.7.2 – uses appropriate procedures for safety in the classroom, home and community MA.B.4.3.2.7.2 – measures accurately with the measurement tools to the specified degree of acc ...
... SC.A.2.3.2.7.3 – determines the mass number and atomic number of an atom from the number of protons and neutrons SC.H.3.3.1.7.2 – uses appropriate procedures for safety in the classroom, home and community MA.B.4.3.2.7.2 – measures accurately with the measurement tools to the specified degree of acc ...
Chapter 2 - HCC Learning Web
... (b) CoCl2 is a compound composed of a metal (left side of periodic table) and nonmetal (right side of the periodic table); therefore, it is an ionic compound. (c) Nitrogen is an element that is listed as diatomic in Table 5.2; therefore, it is a molecular element. (d) SO2 is a compound composed of t ...
... (b) CoCl2 is a compound composed of a metal (left side of periodic table) and nonmetal (right side of the periodic table); therefore, it is an ionic compound. (c) Nitrogen is an element that is listed as diatomic in Table 5.2; therefore, it is a molecular element. (d) SO2 is a compound composed of t ...
Answer - Test Bank 1
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
... 59. (T/F) Chemical compounds are composed of atoms of different elements combined in specific ratios, such as HO1/2. F 60. (T/F) A force called a covalent bond holds the atoms in a molecule together. T 61. (T/F) Atoms of the same element can possess different masses. T 62. (T/F) Cations and anions d ...
Student Copy Study Guide Introduction to Periodic
... 19.The atomic number of an element is the total number of which particles in the nucleus? a. neutrons b. protons c. electrons d. protons and electrons 20. Who was the man who lived from 460 B.C.–370 B.C. and was among the first to suggest the idea of atoms? a. Atomos b. Dalton c. Democritus d. Thoms ...
... 19.The atomic number of an element is the total number of which particles in the nucleus? a. neutrons b. protons c. electrons d. protons and electrons 20. Who was the man who lived from 460 B.C.–370 B.C. and was among the first to suggest the idea of atoms? a. Atomos b. Dalton c. Democritus d. Thoms ...
Redox Introduction
... In burning, oxygen unites rapidly with carbon to form CO2. Observation of these reactions gave rise to the terms "slow" and "rapid" oxidation. Chemists recognize, however, that other nonmetallic elements unite with substances in a manner similar to that of oxygen. – Hydrogen, antimony, and sodium al ...
... In burning, oxygen unites rapidly with carbon to form CO2. Observation of these reactions gave rise to the terms "slow" and "rapid" oxidation. Chemists recognize, however, that other nonmetallic elements unite with substances in a manner similar to that of oxygen. – Hydrogen, antimony, and sodium al ...
Section 4.6 Introduction to the Modern Concept of Atomic Structure
... The pesticide known as DDT paralyzes insects by binding to their nerve cells, leading to uncontrolled firing of the nerves. Before most uses of DDT were banned in the U.S., many insects had developed a resistance to it. Write out the formula for DDT. It contains 14 carbon atoms, 9 hydrogen atoms, an ...
... The pesticide known as DDT paralyzes insects by binding to their nerve cells, leading to uncontrolled firing of the nerves. Before most uses of DDT were banned in the U.S., many insects had developed a resistance to it. Write out the formula for DDT. It contains 14 carbon atoms, 9 hydrogen atoms, an ...
Isotopes - Ms. Bergman`s Classes at DCIS Montbello
... Take 2 mins to SILENTLY read the objective and ...
... Take 2 mins to SILENTLY read the objective and ...
4.6 Oxidation-Reduction (Redox) Reactions Oxidation Reduction
... can be broken down into two half reactions: oxidation (loss of e-): Zn (s) ...
... can be broken down into two half reactions: oxidation (loss of e-): Zn (s) ...
Problems - El Camino College
... c) The mass or an electron 1s about the same as the mass ofa proton. d) There are subatom ic particles in addition to the electron, proton. and neutron. e) The mass of an atom i~ uniformly distributed throughout the atom. f) Most of the particles fucd i11to the gold foi l in the Rutherford experimen ...
... c) The mass or an electron 1s about the same as the mass ofa proton. d) There are subatom ic particles in addition to the electron, proton. and neutron. e) The mass of an atom i~ uniformly distributed throughout the atom. f) Most of the particles fucd i11to the gold foi l in the Rutherford experimen ...
Spectroscopy In Oceanography
... In 1882 Manet predicted that all elements would be found in sea-water. K, Na, Ca, Mg, Sand Cl had been identified in sea-water by 1819, Br in 1826, Bin 1853, Sr and F in 1865. The development of the optical emission spectrograph by Bunsen and Kirchoff in 1860 paved the way to the identification of o ...
... In 1882 Manet predicted that all elements would be found in sea-water. K, Na, Ca, Mg, Sand Cl had been identified in sea-water by 1819, Br in 1826, Bin 1853, Sr and F in 1865. The development of the optical emission spectrograph by Bunsen and Kirchoff in 1860 paved the way to the identification of o ...
CHEMISTRY 123-07 Midterm #1 – Answer key October 14, 2010
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
... PART II: SHORT ANSWER (Each short answer question has a 1-point value!!) 31. Molarity is defined as the number of moles of solute per volume of solution in liters. 32. Ions that contain atoms of more than one element are called polyatomic ions. 33. Proton donors are known as Brønsted acids. 34. A co ...
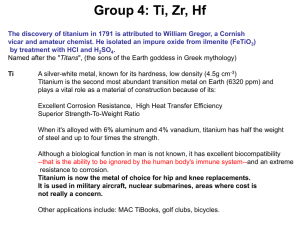
Reaction of niobium with water
... (credited to D. Coster and G. von Hevesey), Hafnium was thought to be present in various minerals and concentrations. It was originally separated from zirconium by repeated recrystallization of the double ammonium or potassium fluorides. Metallic hafnium was first prepared by van Arkel and deBoer by ...
... (credited to D. Coster and G. von Hevesey), Hafnium was thought to be present in various minerals and concentrations. It was originally separated from zirconium by repeated recrystallization of the double ammonium or potassium fluorides. Metallic hafnium was first prepared by van Arkel and deBoer by ...