THE ATOM - Montgomery College
... and cathode because it has a high atomic number A high atomic number means more energy is required to ionize the material Recall that ionization causes eventual breakdown of material ...
... and cathode because it has a high atomic number A high atomic number means more energy is required to ionize the material Recall that ionization causes eventual breakdown of material ...
32 The Atom and the Quantum Answers and Solutions for Chapter
... circumferences, and also the radii of orbits are discrete. Since energy depends upon this radial distance, the energy values are also discrete. (In a more refined wave model, there are standing waves in the radial as well as the orbital direction.) ...
... circumferences, and also the radii of orbits are discrete. Since energy depends upon this radial distance, the energy values are also discrete. (In a more refined wave model, there are standing waves in the radial as well as the orbital direction.) ...
Jan. 26: Symmetries - Michigan State University
... charged pion. In this detector the Ke3 decay leads to a distribution in m* ranging from 280 MeV to -536 MeV; the K&3, from 280 to -516; and the K&3, from 280 to 363 MeV. We emphasize that m* equal to the E' mass is not a preferred result when the three-body decays are analyzed in this way. In additi ...
... charged pion. In this detector the Ke3 decay leads to a distribution in m* ranging from 280 MeV to -536 MeV; the K&3, from 280 to -516; and the K&3, from 280 to 363 MeV. We emphasize that m* equal to the E' mass is not a preferred result when the three-body decays are analyzed in this way. In additi ...
Uniform electric fields - Tasker Milward Physics Website
... γ = Lorentz factor v = velocity c = speed of light You should not need this – you *must* learn to rearrange it yourself!!! ...
... γ = Lorentz factor v = velocity c = speed of light You should not need this – you *must* learn to rearrange it yourself!!! ...
GCSE C1.1 PPT Structure of atoms - School
... Identify each of the three subatomic particles – protons, neutrons and electrons Recall location, mass and charge of each of the three subatomic particles Identify the numbers of protons, neutrons and electrons for each element in the periodic table up to atomic number 20 – calcium ...
... Identify each of the three subatomic particles – protons, neutrons and electrons Recall location, mass and charge of each of the three subatomic particles Identify the numbers of protons, neutrons and electrons for each element in the periodic table up to atomic number 20 – calcium ...
穨 Ams1a
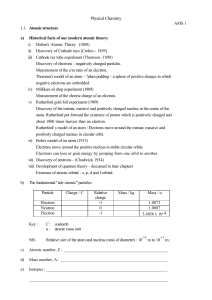
... atom. Rutherford put forward the existence of proton which is positively charged and about 1800 times heavier than an electron. Rutherford' s model of an atom : Electrons move around the minute, massive and positively charged nucleus in circular orbit. vi) Bohr's model of an atom (1913) Electrons mo ...
... atom. Rutherford put forward the existence of proton which is positively charged and about 1800 times heavier than an electron. Rutherford' s model of an atom : Electrons move around the minute, massive and positively charged nucleus in circular orbit. vi) Bohr's model of an atom (1913) Electrons mo ...
Force on a Charged Particle
... Force on a Charged Particle • Charged particles are not confined to a wire. • Cathode-ray tube in monitors and TVs use magnetic fields to deflect electrons to form pictures on the screen. • The screen in coated with phosphor to glow when an electron hits it. ...
... Force on a Charged Particle • Charged particles are not confined to a wire. • Cathode-ray tube in monitors and TVs use magnetic fields to deflect electrons to form pictures on the screen. • The screen in coated with phosphor to glow when an electron hits it. ...
Alpha particle – a positively charged atom that is released in the
... 2. Background radiation – the nuclear radiation that arises naturally from cosmic rays and from radioactive isotopes in the soil and air 3. Beta particle – a charged electron emitted during certain types of radioactive decay, such as beta decay 4. Critical mass – the minimum mass of a fissionable is ...
... 2. Background radiation – the nuclear radiation that arises naturally from cosmic rays and from radioactive isotopes in the soil and air 3. Beta particle – a charged electron emitted during certain types of radioactive decay, such as beta decay 4. Critical mass – the minimum mass of a fissionable is ...
STRUCTURE OF ATOMS
... Neutrons are particles that have the same mass (it's just slightly different) as protons, but have no charge. They contribute only in a minor way to the chemistry of atoms, but they do contribute to the mass, and are important for other reasons that we shall see. Protons and neutrons compose the nu ...
... Neutrons are particles that have the same mass (it's just slightly different) as protons, but have no charge. They contribute only in a minor way to the chemistry of atoms, but they do contribute to the mass, and are important for other reasons that we shall see. Protons and neutrons compose the nu ...
History of the Atom
... When these forces are equal Bqv=qE Then v = E/B When electric field removed, particles given centripetal force by magnetic field Bqv = mv2/r Solved for mass/charge ratio: m/q = Br/v Thomson calculated m/q as 5.686 x 10-12 kg/C ...
... When these forces are equal Bqv=qE Then v = E/B When electric field removed, particles given centripetal force by magnetic field Bqv = mv2/r Solved for mass/charge ratio: m/q = Br/v Thomson calculated m/q as 5.686 x 10-12 kg/C ...
Deflection of Beta Particles in Magnetic Field
... this constant force perpendicular to the velocity vector. This force to change the direction of charged particles and follow a circular path at constant velocity in the magnetic field. So that the magnetic field cause Beta particles to change direction as the particles cross this field. ...
... this constant force perpendicular to the velocity vector. This force to change the direction of charged particles and follow a circular path at constant velocity in the magnetic field. So that the magnetic field cause Beta particles to change direction as the particles cross this field. ...
OscSNS: Precision Neutrino Measurements at
... The flagship cross section analyses of OscSNS are the elastic scattering νee-→ νee- (NC and CC), NC νµe-→ νµe-, NC anti-νµe-→ antiνµe-, NC νµC→ νµC, and the CC νe12C→e-12N interactions. The current world's best measurement of the νee-→ νee- interaction arises in a sample of only 191 events [6], and ...
... The flagship cross section analyses of OscSNS are the elastic scattering νee-→ νee- (NC and CC), NC νµe-→ νµe-, NC anti-νµe-→ antiνµe-, NC νµC→ νµC, and the CC νe12C→e-12N interactions. The current world's best measurement of the νee-→ νee- interaction arises in a sample of only 191 events [6], and ...
Assignment
... An electron enters a uniform electric field produced by applying a potential difference of 150 V between two oppositely charged parallel plates in a vacuum. The plates are separated by a distance d = 0.050 m and are of length L = 0.100 m. The initial velocity of the electron is 1.0 × 107 ms-1 parall ...
... An electron enters a uniform electric field produced by applying a potential difference of 150 V between two oppositely charged parallel plates in a vacuum. The plates are separated by a distance d = 0.050 m and are of length L = 0.100 m. The initial velocity of the electron is 1.0 × 107 ms-1 parall ...
Lepton
A lepton is an elementary, half-integer spin (spin 1⁄2) particle that does not undergo strong interactions, but is subject to the Pauli exclusion principle. The best known of all leptons is the electron, which is directly tied to all chemical properties. Two main classes of leptons exist: charged leptons (also known as the electron-like leptons), and neutral leptons (better known as neutrinos). Charged leptons can combine with other particles to form various composite particles such as atoms and positronium, while neutrinos rarely interact with anything, and are consequently rarely observed.There are six types of leptons, known as flavours, forming three generations. The first generation is the electronic leptons, comprising the electron (e−) and electron neutrino (νe); the second is the muonic leptons, comprising the muon (μ−) and muon neutrino (νμ); and the third is the tauonic leptons, comprising the tau (τ−) and the tau neutrino (ντ). Electrons have the least mass of all the charged leptons. The heavier muons and taus will rapidly change into electrons through a process of particle decay: the transformation from a higher mass state to a lower mass state. Thus electrons are stable and the most common charged lepton in the universe, whereas muons and taus can only be produced in high energy collisions (such as those involving cosmic rays and those carried out in particle accelerators).Leptons have various intrinsic properties, including electric charge, spin, and mass. Unlike quarks however, leptons are not subject to the strong interaction, but they are subject to the other three fundamental interactions: gravitation, electromagnetism (excluding neutrinos, which are electrically neutral), and the weak interaction. For every lepton flavor there is a corresponding type of antiparticle, known as antilepton, that differs from the lepton only in that some of its properties have equal magnitude but opposite sign. However, according to certain theories, neutrinos may be their own antiparticle, but it is not currently known whether this is the case or not.The first charged lepton, the electron, was theorized in the mid-19th century by several scientists and was discovered in 1897 by J. J. Thomson. The next lepton to be observed was the muon, discovered by Carl D. Anderson in 1936, which was classified as a meson at the time. After investigation, it was realized that the muon did not have the expected properties of a meson, but rather behaved like an electron, only with higher mass. It took until 1947 for the concept of ""leptons"" as a family of particle to be proposed. The first neutrino, the electron neutrino, was proposed by Wolfgang Pauli in 1930 to explain certain characteristics of beta decay. It was first observed in the Cowan–Reines neutrino experiment conducted by Clyde Cowan and Frederick Reines in 1956. The muon neutrino was discovered in 1962 by Leon M. Lederman, Melvin Schwartz and Jack Steinberger, and the tau discovered between 1974 and 1977 by Martin Lewis Perl and his colleagues from the Stanford Linear Accelerator Center and Lawrence Berkeley National Laboratory. The tau neutrino remained elusive until July 2000, when the DONUT collaboration from Fermilab announced its discovery.Leptons are an important part of the Standard Model. Electrons are one of the components of atoms, alongside protons and neutrons. Exotic atoms with muons and taus instead of electrons can also be synthesized, as well as lepton–antilepton particles such as positronium.