Atomic Theory History Chem 11
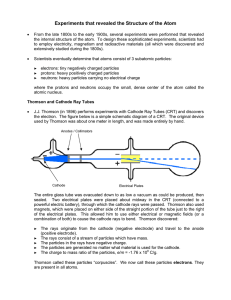
... • Thomson’s model of the atom was called the PLUM PUDDING model because he used a sphere with subatomic particles embedded: negatively charged electrons & positively charged protons dispersed to give an overall neutral charge ...
... • Thomson’s model of the atom was called the PLUM PUDDING model because he used a sphere with subatomic particles embedded: negatively charged electrons & positively charged protons dispersed to give an overall neutral charge ...
teachers.sd43.bc.ca
... • Thomson’s model of the atom was called the PLUM PUDDING model because he used a sphere with subatomic particles embedded: negatively charged electrons & positively charged protons dispersed to give an overall neutral charge ...
... • Thomson’s model of the atom was called the PLUM PUDDING model because he used a sphere with subatomic particles embedded: negatively charged electrons & positively charged protons dispersed to give an overall neutral charge ...
Document
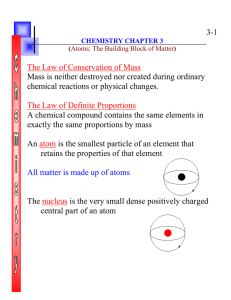
... all atoms of a given element are identical to one another but different from atoms of all other elements atoms of one element cannot be changed into another, created or destroyed compounds are formed when atoms combine in new ways Dalton’s atomic theory explained - law of constant composition ...
... all atoms of a given element are identical to one another but different from atoms of all other elements atoms of one element cannot be changed into another, created or destroyed compounds are formed when atoms combine in new ways Dalton’s atomic theory explained - law of constant composition ...
國立彰化師範大學八十八學年度碩士班招生考試試題
... 1. A projectile is launched at angle /4 from a cliff of height H above sea level. If it falls into the sea at a distance equal to 3H from the base of the cliff, then (a) what is the maximum height above the sea level? (b) what is the time of flight? 2. (a) A non-conducting thin circular disk of rad ...
... 1. A projectile is launched at angle /4 from a cliff of height H above sea level. If it falls into the sea at a distance equal to 3H from the base of the cliff, then (a) what is the maximum height above the sea level? (b) what is the time of flight? 2. (a) A non-conducting thin circular disk of rad ...
Quiz 3-6 fy13 - Nuclear Chemistry practice
... What thickness of what material is the minimum necessary to stop a beta particle? A. three feet of concrete B. three inches of lead C. sheet of aluminum foil D. sheet of paper E. beta particles cannot be stopped ...
... What thickness of what material is the minimum necessary to stop a beta particle? A. three feet of concrete B. three inches of lead C. sheet of aluminum foil D. sheet of paper E. beta particles cannot be stopped ...
Elementary Particle Physics
... We normally use a notation very much like for chemical reactions to indicate particle reactions, for example the process of an electron type neutrino colliding with a neutron to create a proton and an electron: νe + n → p + e− There are two important facts about reactions between elementary particle ...
... We normally use a notation very much like for chemical reactions to indicate particle reactions, for example the process of an electron type neutrino colliding with a neutron to create a proton and an electron: νe + n → p + e− There are two important facts about reactions between elementary particle ...
Perpetual Visualization of Particle Motion and
... Known as a gas, liquid, or plasma Physical phenomena that are well suited to particle system modeling Specifically interested in motion ...
... Known as a gas, liquid, or plasma Physical phenomena that are well suited to particle system modeling Specifically interested in motion ...
particle physics
... Classical particles obey Maxwell-Boltzmann statistics but quantum particles are indistinguishable In quantum mechanics particles are described by a field The probability of finding a particle is ||2 ...
... Classical particles obey Maxwell-Boltzmann statistics but quantum particles are indistinguishable In quantum mechanics particles are described by a field The probability of finding a particle is ||2 ...
d. If the magnetic field remains unchanged, what could be done to
... A) Speed up B) Slow down C) Experience no change in velocity D) Follow a parabolic arc E) Follow a circular arc 2. A charged particle with constant velocity enters a uniform magnetic field whose direction is parallel to the particle’s velocity. The particle will A) speed up B) slow down C) experienc ...
... A) Speed up B) Slow down C) Experience no change in velocity D) Follow a parabolic arc E) Follow a circular arc 2. A charged particle with constant velocity enters a uniform magnetic field whose direction is parallel to the particle’s velocity. The particle will A) speed up B) slow down C) experienc ...
習題六 25.41. (a) The potential on the x axis is (b) The potential on
... the electric field between the plates as being external, and we take the system to be the electron alone. In that ...
... the electric field between the plates as being external, and we take the system to be the electron alone. In that ...
Ch. 19: CQ 4, 18, Pr. 9, 11, 15, 17, 28, 31, 39, 41, 43, 89
... magnitude of the acceleration of the electrons while in the field? (c) What is the speed of the electrons after they travel 4.0 mm through the magnetic field? (d) What strength electric field would give the electrons the same magnitude acceleration as in (b)? (e) Why do we have to use an electric fi ...
... magnitude of the acceleration of the electrons while in the field? (c) What is the speed of the electrons after they travel 4.0 mm through the magnetic field? (d) What strength electric field would give the electrons the same magnitude acceleration as in (b)? (e) Why do we have to use an electric fi ...
(Electrostatics) Posted 07/15/2005
... 1.) How many electrons are on a charged comb which attracts a 1 g piece of paper from a distance of 5 cm away, with an acceleration of 1 cm / s 2 ? Assume that the charge on the comb is equal in magnitude (but of opposite sign!) to that on the paper. Ignore gravity. 2.)Compare the strengths of the e ...
... 1.) How many electrons are on a charged comb which attracts a 1 g piece of paper from a distance of 5 cm away, with an acceleration of 1 cm / s 2 ? Assume that the charge on the comb is equal in magnitude (but of opposite sign!) to that on the paper. Ignore gravity. 2.)Compare the strengths of the e ...
Chapter 4 Assessment Key: 83, 85-89, 106
... , mass number decreases by 4; , no change in mass number; , no change in mass number 86. What is the primary factor determining whether a nucleus is stable or unstable? the neutron-to-proton ratio 87. Explain how energy loss and nuclear stability are related to radioactive decay. Radioactivity re ...
... , mass number decreases by 4; , no change in mass number; , no change in mass number 86. What is the primary factor determining whether a nucleus is stable or unstable? the neutron-to-proton ratio 87. Explain how energy loss and nuclear stability are related to radioactive decay. Radioactivity re ...
Lepton
A lepton is an elementary, half-integer spin (spin 1⁄2) particle that does not undergo strong interactions, but is subject to the Pauli exclusion principle. The best known of all leptons is the electron, which is directly tied to all chemical properties. Two main classes of leptons exist: charged leptons (also known as the electron-like leptons), and neutral leptons (better known as neutrinos). Charged leptons can combine with other particles to form various composite particles such as atoms and positronium, while neutrinos rarely interact with anything, and are consequently rarely observed.There are six types of leptons, known as flavours, forming three generations. The first generation is the electronic leptons, comprising the electron (e−) and electron neutrino (νe); the second is the muonic leptons, comprising the muon (μ−) and muon neutrino (νμ); and the third is the tauonic leptons, comprising the tau (τ−) and the tau neutrino (ντ). Electrons have the least mass of all the charged leptons. The heavier muons and taus will rapidly change into electrons through a process of particle decay: the transformation from a higher mass state to a lower mass state. Thus electrons are stable and the most common charged lepton in the universe, whereas muons and taus can only be produced in high energy collisions (such as those involving cosmic rays and those carried out in particle accelerators).Leptons have various intrinsic properties, including electric charge, spin, and mass. Unlike quarks however, leptons are not subject to the strong interaction, but they are subject to the other three fundamental interactions: gravitation, electromagnetism (excluding neutrinos, which are electrically neutral), and the weak interaction. For every lepton flavor there is a corresponding type of antiparticle, known as antilepton, that differs from the lepton only in that some of its properties have equal magnitude but opposite sign. However, according to certain theories, neutrinos may be their own antiparticle, but it is not currently known whether this is the case or not.The first charged lepton, the electron, was theorized in the mid-19th century by several scientists and was discovered in 1897 by J. J. Thomson. The next lepton to be observed was the muon, discovered by Carl D. Anderson in 1936, which was classified as a meson at the time. After investigation, it was realized that the muon did not have the expected properties of a meson, but rather behaved like an electron, only with higher mass. It took until 1947 for the concept of ""leptons"" as a family of particle to be proposed. The first neutrino, the electron neutrino, was proposed by Wolfgang Pauli in 1930 to explain certain characteristics of beta decay. It was first observed in the Cowan–Reines neutrino experiment conducted by Clyde Cowan and Frederick Reines in 1956. The muon neutrino was discovered in 1962 by Leon M. Lederman, Melvin Schwartz and Jack Steinberger, and the tau discovered between 1974 and 1977 by Martin Lewis Perl and his colleagues from the Stanford Linear Accelerator Center and Lawrence Berkeley National Laboratory. The tau neutrino remained elusive until July 2000, when the DONUT collaboration from Fermilab announced its discovery.Leptons are an important part of the Standard Model. Electrons are one of the components of atoms, alongside protons and neutrons. Exotic atoms with muons and taus instead of electrons can also be synthesized, as well as lepton–antilepton particles such as positronium.