ENERGY!
... energy by collisions between particles in matter. A metal pan on a hot stove. Radiation is the transfer of thermal energy by electromagnetic waves. The heat coming off of a hot stove. Convection is the transfer of thermal energy by the movement of particles from one part of a material to another ...
... energy by collisions between particles in matter. A metal pan on a hot stove. Radiation is the transfer of thermal energy by electromagnetic waves. The heat coming off of a hot stove. Convection is the transfer of thermal energy by the movement of particles from one part of a material to another ...
Notes
... other forms of energy such as sound and heat. The water that runs over the dam might be used to power an electric generator and thus the mechanical energy associated with the water can be transformed into electrical energy. The water was behind the dam because the energy from the sun evaporated ...
... other forms of energy such as sound and heat. The water that runs over the dam might be used to power an electric generator and thus the mechanical energy associated with the water can be transformed into electrical energy. The water was behind the dam because the energy from the sun evaporated ...
Energy Makes it Go!!
... • As we mentioned before we call mechanical energy of a system the sum of its kinetic and potential energy. E = K.E + P.E. • If there are no frictional forces, i.e. losses of energy into heat mechanical energy is conserved • MECHANICAL ENERGY CONSERVED (stays the same) ...
... • As we mentioned before we call mechanical energy of a system the sum of its kinetic and potential energy. E = K.E + P.E. • If there are no frictional forces, i.e. losses of energy into heat mechanical energy is conserved • MECHANICAL ENERGY CONSERVED (stays the same) ...
Conceptual Questions Chap. 13
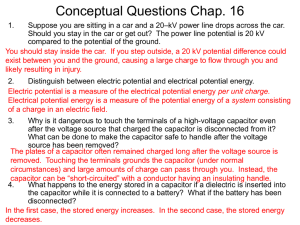
... Why is it dangerous to touch the terminals of a high-voltage capacitor even after the voltage source that charged the capacitor is disconnected from it? What can be done to make the capacitor safe to handle after the voltage source has been removed? The plates of a capacitor often remained charged l ...
... Why is it dangerous to touch the terminals of a high-voltage capacitor even after the voltage source that charged the capacitor is disconnected from it? What can be done to make the capacitor safe to handle after the voltage source has been removed? The plates of a capacitor often remained charged l ...
MSE 156 - Solar Cells, Fuel Cells and Batteries: Materials for the
... The first law of thermodynamics says that in all processes, energy is conserved; neither created or destroyed (must include mass energy if considering nuclear processes). However, the second law of thermodynamics says that in converting from one form of energy to another, the useful output is always ...
... The first law of thermodynamics says that in all processes, energy is conserved; neither created or destroyed (must include mass energy if considering nuclear processes). However, the second law of thermodynamics says that in converting from one form of energy to another, the useful output is always ...
Forms of Energy
... Thermal Energy, or heat, is the vibration and movement of the atoms and molecules within substances. As an object is heated Mechanical Energy is energy stored in objects by tension. up, its atoms and molecules move and collide faster. Compressed springs and stretched rubber bands are Geothermal ener ...
... Thermal Energy, or heat, is the vibration and movement of the atoms and molecules within substances. As an object is heated Mechanical Energy is energy stored in objects by tension. up, its atoms and molecules move and collide faster. Compressed springs and stretched rubber bands are Geothermal ener ...
Energy Notes (part 1)
... as it is heated (the heat energy is not increasing the kinetic energy of the atoms or molecules) In order for a change of state to occur from solid to liquid, or from liquid to gas, the substance gain heat must _____________________________ In order for a change of state to occur from a gas to liqui ...
... as it is heated (the heat energy is not increasing the kinetic energy of the atoms or molecules) In order for a change of state to occur from solid to liquid, or from liquid to gas, the substance gain heat must _____________________________ In order for a change of state to occur from a gas to liqui ...
What is Energy? PPT.
... When you talk on the phone, your voice is transformed into electrical energy, which passes over wires (or is transmitted through the air). The phone on the other end changes the electrical energy into sound energy through the speaker. A car uses stored chemical energy in gasoline to move. The engine ...
... When you talk on the phone, your voice is transformed into electrical energy, which passes over wires (or is transmitted through the air). The phone on the other end changes the electrical energy into sound energy through the speaker. A car uses stored chemical energy in gasoline to move. The engine ...
notes on "Kinetic vs. Potential Energy."
... Nuclear Energy: energy related to the structure of atoms. Whereas electrons are on the outside of an atom’s nucleus, the nucleus is made up of protons and neutrons. The nucleus of an atom contains a huge amount of potential energy. Protons all have the same charge and tend to push away from each ot ...
... Nuclear Energy: energy related to the structure of atoms. Whereas electrons are on the outside of an atom’s nucleus, the nucleus is made up of protons and neutrons. The nucleus of an atom contains a huge amount of potential energy. Protons all have the same charge and tend to push away from each ot ...
Work
... Answer: The man did no work on the car since d=0. He may have burned calories, converting chemical energy into heat, but still, the car did not move. ...
... Answer: The man did no work on the car since d=0. He may have burned calories, converting chemical energy into heat, but still, the car did not move. ...
Thermo I
... The amount of heat Q needed for a certain temperature change ΔT is proportional to the temperature change and to the number of moles n of the substance ...
... The amount of heat Q needed for a certain temperature change ΔT is proportional to the temperature change and to the number of moles n of the substance ...
Conservation of energy

In physics, the law of conservation of energy states that the total energy of an isolated system remains constant—it is said to be conserved over time. Energy can be neither created nor be destroyed, but it transforms from one form to another, for instance chemical energy can be converted to kinetic energy in the explosion of a stick of dynamite.A consequence of the law of conservation of energy is that a perpetual motion machine of the first kind cannot exist. That is to say, no system without an external energy supply can deliver an unlimited amount of energy to its surroundings.