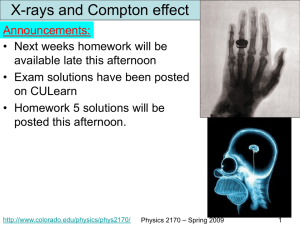
File
... Quantum-Mechanical Model of the Atom • Orbital = region around nucleus where an electron with a given energy level will probably (90%) be found • Four kinds of orbitals s - spherical in shape, lowest orbital for every energy level p - dumbbell shaped, second orbital d - complex “flower” shape, t ...
... Quantum-Mechanical Model of the Atom • Orbital = region around nucleus where an electron with a given energy level will probably (90%) be found • Four kinds of orbitals s - spherical in shape, lowest orbital for every energy level p - dumbbell shaped, second orbital d - complex “flower” shape, t ...
Exam 3 review
... If not looking, then electrons are waves … like wave of fluffy cloud. As soon as we look for an electron, they are like hard balls. Each electron goes through both slits … even though it has mass. (SEEMS TOTALLY WEIRD! Because different than our experience. Size scale of things we perceive) If all y ...
... If not looking, then electrons are waves … like wave of fluffy cloud. As soon as we look for an electron, they are like hard balls. Each electron goes through both slits … even though it has mass. (SEEMS TOTALLY WEIRD! Because different than our experience. Size scale of things we perceive) If all y ...
450 AD and Prior Democritus - reich
... streams of electrons. Goldstein called these positive rays "Kanalstrahlen" - canal rays because it looks like they are passing through a canal. In 1907 a study of how this "ray" was deflected in a ...
... streams of electrons. Goldstein called these positive rays "Kanalstrahlen" - canal rays because it looks like they are passing through a canal. In 1907 a study of how this "ray" was deflected in a ...
Algorithms and Architectures for Quantum Computers—I. Chuang
... The first question is primarily experimental. We intend to build a large-scale, reliable quantum computer over the next few decades. Based on our successes with realizing small quantum computers, and after three years of testing, modeling, and planning, we have come to understand how this can be ach ...
... The first question is primarily experimental. We intend to build a large-scale, reliable quantum computer over the next few decades. Based on our successes with realizing small quantum computers, and after three years of testing, modeling, and planning, we have come to understand how this can be ach ...
The polarization of light - along with refraction, diffraction and
... that light behaves like a wave. The wave model of polarization has allowed us to develop and produce such applications as polarized sunglasses, flat screen TV`s and 3-D movies. This session assumes that you are already familiar with polarization and polarizing filters. It also assumes that you are f ...
... that light behaves like a wave. The wave model of polarization has allowed us to develop and produce such applications as polarized sunglasses, flat screen TV`s and 3-D movies. This session assumes that you are already familiar with polarization and polarizing filters. It also assumes that you are f ...
- Lorentz Center
... The Two Steps of the Renormalization Group Step 1: “Integrate out ” or “thin out” degrees of freedom associated with small scales, and absorb contributions into effective interactions between the remaining degrees of freedom. Real-space RG: Elimination of small-wavelength degrees of freedom is carr ...
... The Two Steps of the Renormalization Group Step 1: “Integrate out ” or “thin out” degrees of freedom associated with small scales, and absorb contributions into effective interactions between the remaining degrees of freedom. Real-space RG: Elimination of small-wavelength degrees of freedom is carr ...
4. Structure of the Atom
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
PDF 1
... Consider an electron ‘trapped’ in a potential well of width a = 2 nm. Using standard values for mass of the electron me and h the lowest energy of the electron is given by equation 27 to be 1.51 × 10−20 J or 0.09 eV or 90 meV . This corresponds to n = 1 in equation 27. The energies of electrons in t ...
... Consider an electron ‘trapped’ in a potential well of width a = 2 nm. Using standard values for mass of the electron me and h the lowest energy of the electron is given by equation 27 to be 1.51 × 10−20 J or 0.09 eV or 90 meV . This corresponds to n = 1 in equation 27. The energies of electrons in t ...
Lecture 3: Heterostructures, Quasielectric Fields, and Quantum
... 0 ≤ y ≤ 1, has a bandgap energy Eg in between those of AlAs and GaAs. Grading the composition, that is varying the concentration with position, gives spatially-varying semiconductor properties. The Aly Ga1−y As alloy system The AlAs/GaAs family of materials has been extensively developed for its use ...
... 0 ≤ y ≤ 1, has a bandgap energy Eg in between those of AlAs and GaAs. Grading the composition, that is varying the concentration with position, gives spatially-varying semiconductor properties. The Aly Ga1−y As alloy system The AlAs/GaAs family of materials has been extensively developed for its use ...
The Atom and Its Properties
... Electrons exist only in discrete, “allowable” energy levels • Energy is involved in moving electrons from one energy level to another • Principal quantum number (n) - specifies the electron’s major energy level • The lowest energy is n=1, the next lowest in ...
... Electrons exist only in discrete, “allowable” energy levels • Energy is involved in moving electrons from one energy level to another • Principal quantum number (n) - specifies the electron’s major energy level • The lowest energy is n=1, the next lowest in ...
Atomic 1
... (1900 - 1958) recognized that no two electrons, in the same atom, could have the same set of four quantum numbers. Two electrons may have the same n, l and ml quantum numbers, placing them in the same atomic orbital, but then they must have different spins. Thus each orbital can accommodate two elec ...
... (1900 - 1958) recognized that no two electrons, in the same atom, could have the same set of four quantum numbers. Two electrons may have the same n, l and ml quantum numbers, placing them in the same atomic orbital, but then they must have different spins. Thus each orbital can accommodate two elec ...
The Structure of the Atom [Режим совместимости]
... For example, a subshell with a label of ℓ = 1 is called a “p subshell,” and an orbital found in that subshell is called a “p orbital” ...
... For example, a subshell with a label of ℓ = 1 is called a “p subshell,” and an orbital found in that subshell is called a “p orbital” ...
What`s bad about this habit
... In my youth I had little sympathy for Niels Bohr’s philosophical pronouncements. In a review of Bohr’s philosophical writings I said that “one wants to shake the author vigorously and demand that he explain himself further or at least try harder to paraphrase some of his earlier formulations.” But i ...
... In my youth I had little sympathy for Niels Bohr’s philosophical pronouncements. In a review of Bohr’s philosophical writings I said that “one wants to shake the author vigorously and demand that he explain himself further or at least try harder to paraphrase some of his earlier formulations.” But i ...
Misconception about Quantum Physics slides
... – evolves according to well prescribed rules (Schrödinger eqn.) ...
... – evolves according to well prescribed rules (Schrödinger eqn.) ...
Quantum electrodynamics

In particle physics, quantum electrodynamics (QED) is the relativistic quantum field theory of electrodynamics. In essence, it describes how light and matter interact and is the first theory where full agreement between quantum mechanics and special relativity is achieved. QED mathematically describes all phenomena involving electrically charged particles interacting by means of exchange of photons and represents the quantum counterpart of classical electromagnetism giving a complete account of matter and light interaction.In technical terms, QED can be described as a perturbation theory of the electromagnetic quantum vacuum. Richard Feynman called it ""the jewel of physics"" for its extremely accurate predictions of quantities like the anomalous magnetic moment of the electron and the Lamb shift of the energy levels of hydrogen.

















![The Structure of the Atom [Режим совместимости]](http://s1.studyres.com/store/data/001854271_1-ef1245c1f375ab31b64ec8e56383a02e-300x300.png)