PHYSICAL SETTING CHEMISTRY
... A separate answer sheet for Part A and Part B–1 has been provided to you. Follow the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers ...
... A separate answer sheet for Part A and Part B–1 has been provided to you. Follow the instructions from the proctor for completing the student information on your answer sheet. Record your answers to the Part A and Part B–1 multiple-choice questions on this separate answer sheet. Record your answers ...
UNIT 2 – ATOMIC THEORY AND STRUCTURE
... Another Example - The fictional element J is found to have three naturally occurring isotopes: J-298 has an isotopic mass of 298.01 amu and a relative abundance of 15.53% J-300 has an isotopic mass of 299.98 amu and a relative abundance of 33.35% J-302 has an isotopic mass of 301.99 amu and a relati ...
... Another Example - The fictional element J is found to have three naturally occurring isotopes: J-298 has an isotopic mass of 298.01 amu and a relative abundance of 15.53% J-300 has an isotopic mass of 299.98 amu and a relative abundance of 33.35% J-302 has an isotopic mass of 301.99 amu and a relati ...
Ch 2 Sample Exercises PPT
... Sample Exercise 2.7 Writing Chemical Symbols for Ions Give the chemical symbol, including mass number, for each of the following ions: (a) The ion with 22 protons, 26 neutrons, and 19 electrons; (b) the ion of sulfur that has 16 neutrons and 18 electrons. Solution (a) The number of protons (22) is ...
... Sample Exercise 2.7 Writing Chemical Symbols for Ions Give the chemical symbol, including mass number, for each of the following ions: (a) The ion with 22 protons, 26 neutrons, and 19 electrons; (b) the ion of sulfur that has 16 neutrons and 18 electrons. Solution (a) The number of protons (22) is ...
Experimental and Theoretical Charge Density Analysis of a
... ABSTRACT: Bromoethyl sulfonium trifluoromethanesulfonate is a salt complex in which a sulfur atom makes three covalent bonds. This molecule has been proved to act as an efficient annulation reagent which results in formation of synthetically challenging and pharmaceutically important 4-, 5-, 6-, and 7- ...
... ABSTRACT: Bromoethyl sulfonium trifluoromethanesulfonate is a salt complex in which a sulfur atom makes three covalent bonds. This molecule has been proved to act as an efficient annulation reagent which results in formation of synthetically challenging and pharmaceutically important 4-, 5-, 6-, and 7- ...
Sample Exercise 2.1 Illustrating the Size of an Atom
... Sample Exercise 2.7 Writing Chemical Symbols for Ions Give the chemical symbol, including mass number, for each of the following ions: (a) The ion with 22 protons, 26 neutrons, and 19 electrons; (b) the ion of sulfur that has 16 neutrons and 18 electrons. Solution (a) The number of protons (22) is ...
... Sample Exercise 2.7 Writing Chemical Symbols for Ions Give the chemical symbol, including mass number, for each of the following ions: (a) The ion with 22 protons, 26 neutrons, and 19 electrons; (b) the ion of sulfur that has 16 neutrons and 18 electrons. Solution (a) The number of protons (22) is ...
www.studyguide.pk
... Write your name, Centre number and candidate number on all the work you hand in. Write in dark blue or black pen. You may use a pencil for any diagrams, graphs, or rough working. ...
... Write your name, Centre number and candidate number on all the work you hand in. Write in dark blue or black pen. You may use a pencil for any diagrams, graphs, or rough working. ...
WORD - SSS Chemistry
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
... ___________________________ devised the Scattering Experiment, which showed that all atoms had a small dense __________________________. ...
AP Chemistry
... 1. zero for isolated neutral atom 2. equals ionic charge for monatomic ions b. overall oxidation number for polyatomic species 1. zero for neutral compound or molecule 2. equals ionic charge for polyatomic ion c. assign oxidation numbers to atoms within a molecule or polyatomic ion 1. assign the sta ...
... 1. zero for isolated neutral atom 2. equals ionic charge for monatomic ions b. overall oxidation number for polyatomic species 1. zero for neutral compound or molecule 2. equals ionic charge for polyatomic ion c. assign oxidation numbers to atoms within a molecule or polyatomic ion 1. assign the sta ...
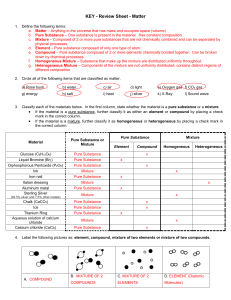
Matter Test Review Sheet
... d. Element – Pure substance composed of only one type of atom. e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Hetero ...
... d. Element – Pure substance composed of only one type of atom. e. Compound – Pure substance composed of 2 or more elements chemically bonded together. Can be broken down by chemical processes. f. Homogeneous Mixture – Substance that make up the mixture are distributed uniformly throughout. g. Hetero ...
Chapter 2 Atoms, Molecules, and Ions
... We will assume that barium and oxygen form ions that have the same number of electrons as the nearest noble-gas atom. From the periodic table, we see that barium has atomic number 56. The nearest noble gas is xenon, atomic number 54. Barium can attain a stable arrangement of 54 electrons by losing t ...
... We will assume that barium and oxygen form ions that have the same number of electrons as the nearest noble-gas atom. From the periodic table, we see that barium has atomic number 56. The nearest noble gas is xenon, atomic number 54. Barium can attain a stable arrangement of 54 electrons by losing t ...
Class_X–Science__term_I
... 1) Chemical reaction:-Chemical reaction is a change in which one or more new substances are formed. 2) Chemical Equations:-Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3) Balanced Chemical equations:-The chemical ...
... 1) Chemical reaction:-Chemical reaction is a change in which one or more new substances are formed. 2) Chemical Equations:-Representation of a chemical reaction in terms of symbols and formulae of the reactants and products is known as chemical equation. 3) Balanced Chemical equations:-The chemical ...
Biochemistry Part A PPT
... • Ions are formed by transfer of valence shell electrons between atoms • Anions (– charge) have gained one or more electrons • Cations (+ charge) have lost one or more electrons ...
... • Ions are formed by transfer of valence shell electrons between atoms • Anions (– charge) have gained one or more electrons • Cations (+ charge) have lost one or more electrons ...
Chemistry 2014 - SC3210 IC Scope and Sequence
... Use the periodic table to determine the number of valence electrons available for bonding. ...
... Use the periodic table to determine the number of valence electrons available for bonding. ...
Electrochemistry - Menihek Home Page
... hydroxide ions (OH-) enable the redox to occur more readily. These ions, as well as water, are used to balance the number of oxygen atoms and hydrogen atoms. This process involves many steps....they must be memorized: To balance redox equations under acidic conditions: ...
... hydroxide ions (OH-) enable the redox to occur more readily. These ions, as well as water, are used to balance the number of oxygen atoms and hydrogen atoms. This process involves many steps....they must be memorized: To balance redox equations under acidic conditions: ...
Thermochemistry
... IEI = 419 kJ mol-1 ΔH°a= 279 kJ mol-1 ΔH°f = -257 kJ mol-1 ΔH°a = 78 kJ mol-1 EAI= -199.5 kJ mol-1 ΔH°lattice = -1979 kJ mol EAII - 648.5 kJ mol-1 ...
... IEI = 419 kJ mol-1 ΔH°a= 279 kJ mol-1 ΔH°f = -257 kJ mol-1 ΔH°a = 78 kJ mol-1 EAI= -199.5 kJ mol-1 ΔH°lattice = -1979 kJ mol EAII - 648.5 kJ mol-1 ...
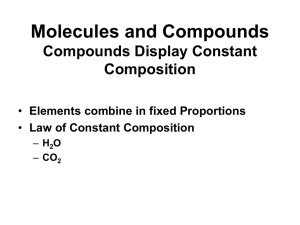
Chemical Reactions
... Word Equations • Word Equations: an equation in which the reactants and products in a chemical reaction are represented by words instead of chemical formulas. • The problem with word equations is they do not actually show the number of atoms or molecules of each substance… formulas would have to be ...
... Word Equations • Word Equations: an equation in which the reactants and products in a chemical reaction are represented by words instead of chemical formulas. • The problem with word equations is they do not actually show the number of atoms or molecules of each substance… formulas would have to be ...
Defining the Atom - Warren County Public Schools
... Teachers open the door, but you must enter by yourself. ...
... Teachers open the door, but you must enter by yourself. ...
Monte Carlo Simulation of Water Radiolysis for
... a Gaussian distribution with a mean displacement of 1.5 nm. The H3O+ is assumed to be at the same position as the H2O+ and the OH radical is positioned with random orientation at a distance of 0.29 nm.26) In the dissociation of an excited water molecule into H and OH radicals the products are assume ...
... a Gaussian distribution with a mean displacement of 1.5 nm. The H3O+ is assumed to be at the same position as the H2O+ and the OH radical is positioned with random orientation at a distance of 0.29 nm.26) In the dissociation of an excited water molecule into H and OH radicals the products are assume ...
1 Structure of Atom
... positrons, neutrinos and antiprotons have been discovered. A great deal of recent research is producing a long list of still other subatomic particles with names quarks, pions and gluons. With each discovery, the picture of atomic structure becomes increasingly complex. Fortunately, the three-partic ...
... positrons, neutrinos and antiprotons have been discovered. A great deal of recent research is producing a long list of still other subatomic particles with names quarks, pions and gluons. With each discovery, the picture of atomic structure becomes increasingly complex. Fortunately, the three-partic ...
5.1 Revising the Atomic Model
... • It could not explain the chemical properties of elements. For example, Rutherford’s model could not explain why an object such as the iron scroll shown here first glows dull red, then yellow, and then white when heated to higher and higher temperatures. ...
... • It could not explain the chemical properties of elements. For example, Rutherford’s model could not explain why an object such as the iron scroll shown here first glows dull red, then yellow, and then white when heated to higher and higher temperatures. ...
chapter 1–introduction to earth history
... Neutron (22): Electrically neutral particles of matter existing along with protons in the atomic nucleus of all elements except the mass 1 isotope of hydrogen. Nuclide (22): The different weight configurations of an element caused by atoms of that element having differing numbers of neutrons. Nuclid ...
... Neutron (22): Electrically neutral particles of matter existing along with protons in the atomic nucleus of all elements except the mass 1 isotope of hydrogen. Nuclide (22): The different weight configurations of an element caused by atoms of that element having differing numbers of neutrons. Nuclid ...
Chapter One
... It seems logical to start a book of this nature with the question: What is chem istry? Most dictionaries define chemistry as the science that deals with the com position, structure, and properties of substances and the reactions by which one substance is converted into another. Knowing the defi ni ...
... It seems logical to start a book of this nature with the question: What is chem istry? Most dictionaries define chemistry as the science that deals with the com position, structure, and properties of substances and the reactions by which one substance is converted into another. Knowing the defi ni ...
What Are Compounds? - Parma School District
... • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers are assigned to the atoms composing the compound or ion. • Unlike ionic charges, oxidation numbers do not have an exact physical meaning: rather, they se ...
... • In order to indicate the general distribution of electrons among the bonded atoms in a molecular compound or a polyatomic ion, oxidation numbers are assigned to the atoms composing the compound or ion. • Unlike ionic charges, oxidation numbers do not have an exact physical meaning: rather, they se ...