2 - TestBankTop
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
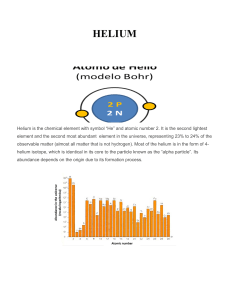
HELIUM - IDC
... observable matter (almost all matter that is not hydrogen). Most of the helium is in the form of 4helium isotope, which is identical in its core to the particle known as the “alpha particle”. Its abundance depends on the origin due to its formation process. ...
... observable matter (almost all matter that is not hydrogen). Most of the helium is in the form of 4helium isotope, which is identical in its core to the particle known as the “alpha particle”. Its abundance depends on the origin due to its formation process. ...
FREE Sample Here
... Full file at http://emailtestbank.com/ Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbell Answer: B Topic: Concept 2.2 Skill: Knowledge/Comprehension 25) Which drawing is of an atom with the atomic number of 6? Answer: A Topic: Concept 2.2 Skill: Knowledge/Comprehension 26) Which d ...
... Full file at http://emailtestbank.com/ Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbell Answer: B Topic: Concept 2.2 Skill: Knowledge/Comprehension 25) Which drawing is of an atom with the atomic number of 6? Answer: A Topic: Concept 2.2 Skill: Knowledge/Comprehension 26) Which d ...
CHEMISTRY 103 – Practice Problems #3 Chapters 8 – 10 http
... a. To reduce lone pair-lone pair electron repulsions in an octahedral electron domain geometry, the first lone pair of electrons are placed in one of the four equatorial positions rather than the axial positions since the six locations are not equivalent. b. The dipole moment, µ, is equal to Qr wher ...
... a. To reduce lone pair-lone pair electron repulsions in an octahedral electron domain geometry, the first lone pair of electrons are placed in one of the four equatorial positions rather than the axial positions since the six locations are not equivalent. b. The dipole moment, µ, is equal to Qr wher ...
Class XI Physical Chemistry Short note
... Until 1920 an atom was supposed to consist of only 2 fundamental particles i.e. electrons and protons. Since electrons have negligible mass, the entire mass of the atom was regarded as the mass of the proton only. Each proton has a mass of 1.67x 10-24 g which is taken as 1 unit mass. In 1920, Ruther ...
... Until 1920 an atom was supposed to consist of only 2 fundamental particles i.e. electrons and protons. Since electrons have negligible mass, the entire mass of the atom was regarded as the mass of the proton only. Each proton has a mass of 1.67x 10-24 g which is taken as 1 unit mass. In 1920, Ruther ...
1999 Advanced Placement Chemistry Exam
... Select the one lettered choice that best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set. Questions 1–4 refer to the following types of energy. (A) (B) (C) (D) (E) ...
... Select the one lettered choice that best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set. Questions 1–4 refer to the following types of energy. (A) (B) (C) (D) (E) ...
Term 111, Final Exam (All correct choices are A): 1. What is the
... A) an element with a large Ei and an element with a small negative Eea B) an element with a small Ei and an element with a small negative Eea C) elements with equal values of Ei and Eea D) an element with a large Ei and an element with a large negative Eea E) an element with a small Ei and an elemen ...
... A) an element with a large Ei and an element with a small negative Eea B) an element with a small Ei and an element with a small negative Eea C) elements with equal values of Ei and Eea D) an element with a large Ei and an element with a large negative Eea E) an element with a small Ei and an elemen ...
Atoms, Molecules, and Ions
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
... atoms being the same (oxygen). Since each "ball" represents an individual atom, the three models on the left can be eliminated since they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two e ...
AP `99 Multiple Choice
... Select the one lettered choice that best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set. Questions 1–4 refer to the following types of energy. (A) (B) (C) (D) (E) ...
... Select the one lettered choice that best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set. Questions 1–4 refer to the following types of energy. (A) (B) (C) (D) (E) ...
Honors Chemistry Curr
... table, developing laboratory techniques, interpreting data, and writing up experiments are stressed. The extensive laboratory work ranges from investigating the fundamental laws of chemical change to the analytical chemistry of acid-base titration and determination of reaction rates. It is expected ...
... table, developing laboratory techniques, interpreting data, and writing up experiments are stressed. The extensive laboratory work ranges from investigating the fundamental laws of chemical change to the analytical chemistry of acid-base titration and determination of reaction rates. It is expected ...
`A` LEVEL H2 CHEMISTRY ORGANIC REACTIONS SUMMARY By
... (a) identify and describe protons, neutrons and electrons in terms of their relative charges and relative masses (b) deduce the behaviour of beams of protons, neutrons and electrons in an electric field (c) describe the distribution of mass and charges within an atom (d) deduce the numbers of proton ...
... (a) identify and describe protons, neutrons and electrons in terms of their relative charges and relative masses (b) deduce the behaviour of beams of protons, neutrons and electrons in an electric field (c) describe the distribution of mass and charges within an atom (d) deduce the numbers of proton ...
Honors Chemistry
... table, developing laboratory techniques, interpreting data, and writing up experiments are stressed. The extensive laboratory work ranges from investigating the fundamental laws of chemical change to the analytical chemistry of acid-base titration and determination of reaction rates. It is expected ...
... table, developing laboratory techniques, interpreting data, and writing up experiments are stressed. The extensive laboratory work ranges from investigating the fundamental laws of chemical change to the analytical chemistry of acid-base titration and determination of reaction rates. It is expected ...
Indian Journal of Chemistry
... a variety of synthetic conditions by polymerizing the respective monomers in the presence of silica prepared in situ from an aqueous sodium silicate solution. The composites, thus synthesized, have been characterized by a wide ...
... a variety of synthetic conditions by polymerizing the respective monomers in the presence of silica prepared in situ from an aqueous sodium silicate solution. The composites, thus synthesized, have been characterized by a wide ...
FREE Sample Here
... A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14. B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7. C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams. D) The nitrogen atom has a mass number o ...
... A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14. B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7. C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams. D) The nitrogen atom has a mass number o ...
FREE Sample Here
... A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14. B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7. C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams. D) The nitrogen atom has a mass number o ...
... A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14. B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7. C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams. D) The nitrogen atom has a mass number o ...
FREE Sample Here
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
... http://testbankwizard.eu/Test-Bank-for-Biology-with-MasteringBiology-8th-Edition-by-Campbe ll 45) Nitrogen (N) is much more electronegative than hydrogen (H). Which of the following statements is correct about the atoms in ammonia (NH3)? A) Each hydrogen atom has a partial positive charge. B) The ni ...
CHEMISTRY SEC 06 SYLLABUS
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
... Preparation of hydrogen from action of dilute non-oxdising acids on certain metals, exemplified by dilute hydrochloric acid or dilute sulfuric acid on magnesium, zinc or iron. Test for hydrogen. Combustion of hydrogen - its advantages and disadvantages as a fuel. Reducing action of hydrogen with met ...
Biology, 8e (Campbell) Chapter 2 The Chemical Context of Life
... A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14. B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7. C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams. D) The nitrogen atom has a mass number o ...
... A) The nitrogen atom has a mass number of approximately 7 daltons and an atomic mass of 14. B) The nitrogen atom has a mass number of approximately 14 daltons and an atomic mass of 7. C) The nitrogen atom has a mass number of 14 and an atomic mass of 7 grams. D) The nitrogen atom has a mass number o ...
WRL0001.tmp - Ethiopian Teachers Association
... lessons., The strategies should challenge preconceptions and school-made misconceptions through recommending alternatives to the traditional approaches, such as setting up simplified laboratory experiments, use of structural models and technology-based methods. Chemistry is one of the most important ...
... lessons., The strategies should challenge preconceptions and school-made misconceptions through recommending alternatives to the traditional approaches, such as setting up simplified laboratory experiments, use of structural models and technology-based methods. Chemistry is one of the most important ...
Exam Review Day - Chemistry with Mr. Saval
... The number of neutrons and protons direclty affect this. ...
... The number of neutrons and protons direclty affect this. ...
Chapter 4 Electron Configurations
... frequencies in the line spectrum of hydrogen correspond to the quantity of nrg emitted when an e- moves from a higher to lower state. ...
... frequencies in the line spectrum of hydrogen correspond to the quantity of nrg emitted when an e- moves from a higher to lower state. ...
1. Which idea of John Dalton is no longer considered part of the
... End of Goal 2 Sample Items In compliance with federal law, including the provisions of Title IX of the Education Amendments of 1972, the Department of Public Instruction does not discriminate on the basis of race, sex, religion, color, national or ethnic origin, age, disability, or military service ...
... End of Goal 2 Sample Items In compliance with federal law, including the provisions of Title IX of the Education Amendments of 1972, the Department of Public Instruction does not discriminate on the basis of race, sex, religion, color, national or ethnic origin, age, disability, or military service ...
Mole - My CCSD
... Is calculated using the atomic mass of each element in the compound, times how many atoms of each element are in the compound. ...
... Is calculated using the atomic mass of each element in the compound, times how many atoms of each element are in the compound. ...
physical change
... useful way to classify matter is based on what makes up the matter. Every sample of matter is either an element, a compound, or a mixture. ...
... useful way to classify matter is based on what makes up the matter. Every sample of matter is either an element, a compound, or a mixture. ...