Chapter 2 – Atoms, Ions, and the Periodic Table
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
FREE Sample Here
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
Chapter 2 – Atoms, Ions, and the Periodic Table
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
... The atomic number of an atom is equal to the number of protons. If you know the name of the element, you can find the atomic number by finding the element on the periodic table. For example, for iron (Fe), you can find the atomic number, 26, listed with the element symbol on the fourth period of the ...
AP Chemistry - cloudfront.net
... 8.37 Which group in the periodic table has elements with high IE1 and very negative first electron affinities (EA1)? What is the charge on the ions that these atoms form? 8.59 Write the charge and full ground-state electron configuration of the monatomic ion most likely to be formed by each of the f ...
... 8.37 Which group in the periodic table has elements with high IE1 and very negative first electron affinities (EA1)? What is the charge on the ions that these atoms form? 8.59 Write the charge and full ground-state electron configuration of the monatomic ion most likely to be formed by each of the f ...
a) How many moles of water are created when 108 moles of oxygen
... work as is explained in the lessons. You are required to have this package completed BEFORE you write your unit test. Do your best and ask questions if you don’t understand anything! ...
... work as is explained in the lessons. You are required to have this package completed BEFORE you write your unit test. Do your best and ask questions if you don’t understand anything! ...
Word - chemmybear.com
... electrode (where K+ is attracted) and have it react with water to form H2 and OH-. K+ + e- K 2K° + 2H2O 2K+ + 2OH- + H2 The combination of these two reactions is exactly what happens when water is reduced at the cathode. 8. (Trick #2) When CuSO4(aq) is electrolyzed, you know that Cu° metal is go ...
... electrode (where K+ is attracted) and have it react with water to form H2 and OH-. K+ + e- K 2K° + 2H2O 2K+ + 2OH- + H2 The combination of these two reactions is exactly what happens when water is reduced at the cathode. 8. (Trick #2) When CuSO4(aq) is electrolyzed, you know that Cu° metal is go ...
The Mole
... The Definition of The Mole The mass of an element or compound that contains as many elementary (representative) particles (atoms, molecules, ions, etc.) as there are atoms in exactly 12 grams of 12C. ...
... The Definition of The Mole The mass of an element or compound that contains as many elementary (representative) particles (atoms, molecules, ions, etc.) as there are atoms in exactly 12 grams of 12C. ...
Chemistry - Summative Practice and Review for Chapter 4 and 5
... ____ 24. How many energy sublevels are in the second principal energy level? a. 1 c. 3 b. 2 d. 4 ____ 25. What is the maximum number of electrons in the second principal energy level? a. 2 c. 18 b. 8 d. 32 ____ 26. What is the number of electrons in the outermost energy level of an oxygen atom? a. 2 ...
... ____ 24. How many energy sublevels are in the second principal energy level? a. 1 c. 3 b. 2 d. 4 ____ 25. What is the maximum number of electrons in the second principal energy level? a. 2 c. 18 b. 8 d. 32 ____ 26. What is the number of electrons in the outermost energy level of an oxygen atom? a. 2 ...
Atomic structure and the periodic tabl
... At KS3 students present the structure of the atom using Dalton’s atomic model. Dalton believed that atoms were tiny indestructible units which were solid particles and this is how it is understood by many students at KS3. At GCSE, students describe the atom as a positively charged nucleus surrounded ...
... At KS3 students present the structure of the atom using Dalton’s atomic model. Dalton believed that atoms were tiny indestructible units which were solid particles and this is how it is understood by many students at KS3. At GCSE, students describe the atom as a positively charged nucleus surrounded ...
Document
... Sample Exercise 2.6 Relating Empirical and Molecular Formulas Write the empirical formulas for (a) glucose, a substance also known as either blood sugar or dextrose, molecular formula C6H12O6; (b) nitrous oxide, a substance used as an anesthetic and commonly called laughing gas, molecular ...
... Sample Exercise 2.6 Relating Empirical and Molecular Formulas Write the empirical formulas for (a) glucose, a substance also known as either blood sugar or dextrose, molecular formula C6H12O6; (b) nitrous oxide, a substance used as an anesthetic and commonly called laughing gas, molecular ...
FREE Sample Here
... 42) How many electrons are involved in a double covalent bond? A) one B) two C) three D) four Answer: D Bloom's Taxonomy: Knowledge/Comprehension Section: 2.3 43) If an atom has a charge of +1, which of the following must be true? A) It has two more protons than neutrons. B) It has the same number o ...
... 42) How many electrons are involved in a double covalent bond? A) one B) two C) three D) four Answer: D Bloom's Taxonomy: Knowledge/Comprehension Section: 2.3 43) If an atom has a charge of +1, which of the following must be true? A) It has two more protons than neutrons. B) It has the same number o ...
student`s book - Macmillan Education South Africa
... All matter is made up of tiny units or particles called atoms. Elements like gold, copper and carbon are made up of atoms. The atom is the smallest part of an element that can exist and still retain the properties of the element. An element is a substance that cannot be broken down into a simpler su ...
... All matter is made up of tiny units or particles called atoms. Elements like gold, copper and carbon are made up of atoms. The atom is the smallest part of an element that can exist and still retain the properties of the element. An element is a substance that cannot be broken down into a simpler su ...
Atoms - ChemGod.com
... Law of Definite Proportions Joseph Proust made a series of mixtures of different elements and discovered that the ratio of the masses that reacted was always the same. For example if mixing hydrogen and oxygen to get water, he found that if you started with 16.0 g of oxygen, you needed 2.0 g of hyd ...
... Law of Definite Proportions Joseph Proust made a series of mixtures of different elements and discovered that the ratio of the masses that reacted was always the same. For example if mixing hydrogen and oxygen to get water, he found that if you started with 16.0 g of oxygen, you needed 2.0 g of hyd ...
Chapter 3
... compounds are made of the same 2 elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole ...
... compounds are made of the same 2 elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole ...
Worksheet Significant Figures
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
Chemistry Standards Clarification
... Compare the strength of the forces of attraction between molecules of different elements. (For example, at room temperature, chlorine is a gas and iodine is a solid.) Predict whether the forces of attraction in a solid are primarily metallic, covalent, network covalent, or ionic based upon the eleme ...
... Compare the strength of the forces of attraction between molecules of different elements. (For example, at room temperature, chlorine is a gas and iodine is a solid.) Predict whether the forces of attraction in a solid are primarily metallic, covalent, network covalent, or ionic based upon the eleme ...
Effects of antioxidants for the degradation of flame
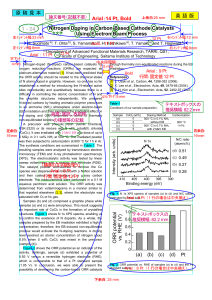
... was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity was tested by linear sweep voltamme ...
... was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electrocatalytic activity was tested by linear sweep voltamme ...
what`s ahead - Al Akhawayn University
... What happens to most of the a particles that strike the gold foil in Rutherford’s experiment? Why do they behave that way? ...
... What happens to most of the a particles that strike the gold foil in Rutherford’s experiment? Why do they behave that way? ...
Lecture 7. Fundamentals of atmospheric chemistry: Part 2 1
... These terms are sometimes confusing since the reduction process involves adding an electron. Keep in mind it's the charge that's being reduced in this case. Oxidation receives its name because almost all reactions with oxygen involve some other element losing electrons to the oxygen. Only fluorine w ...
... These terms are sometimes confusing since the reduction process involves adding an electron. Keep in mind it's the charge that's being reduced in this case. Oxidation receives its name because almost all reactions with oxygen involve some other element losing electrons to the oxygen. Only fluorine w ...
401
... by Eqs. (5) and (7) implies that each electron is captured essentially within the exponential region around the nucleus, because the exponential function decays most rapidly among many other functions. This is a theoretical origin of the local concept in chemistry, which is supported also by the suc ...
... by Eqs. (5) and (7) implies that each electron is captured essentially within the exponential region around the nucleus, because the exponential function decays most rapidly among many other functions. This is a theoretical origin of the local concept in chemistry, which is supported also by the suc ...
chemistry
... arranged in order of increasing (1) atomic mass (3) mass number (2) atomic number (4) oxidation number 2 Which particle has a mass that is approximately the same as the mass of a proton? (1) an alpha particle (3) a neutron (2) a beta particle (4) a positron 3 An atom of an element forms a 2+ ion. In ...
... arranged in order of increasing (1) atomic mass (3) mass number (2) atomic number (4) oxidation number 2 Which particle has a mass that is approximately the same as the mass of a proton? (1) an alpha particle (3) a neutron (2) a beta particle (4) a positron 3 An atom of an element forms a 2+ ion. In ...