Lesson 1 | Discovering Parts of an Atom
... Discovering Parts of an Atom A. Early Ideas About Matter 1. Many ancient Greek philosophers, such as Aristotle, thought that all matter was made of only four elements—fire, water, air, and ...
... Discovering Parts of an Atom A. Early Ideas About Matter 1. Many ancient Greek philosophers, such as Aristotle, thought that all matter was made of only four elements—fire, water, air, and ...
Slide 1
... Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements ...
... Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements ...
History of the Atom
... o Atoms of the same element are exactly alike o Atoms join with other atoms to make new substances ...
... o Atoms of the same element are exactly alike o Atoms join with other atoms to make new substances ...
Chapter 5 Notes
... designated them using integers called _________________________ numbers. 16. The first quantum number is the ______________________ quantum number (n). It is the same as the row number of the element. The higher the energy level the ___________________ the energy. 17. Each energy level consists of e ...
... designated them using integers called _________________________ numbers. 16. The first quantum number is the ______________________ quantum number (n). It is the same as the row number of the element. The higher the energy level the ___________________ the energy. 17. Each energy level consists of e ...
ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
Nuclear radiation 4
... Nuclear Radiation Ex4 1. In the reactor of a nuclear power station, neutrons split uranium nuclei to produce heat in what is known as a “chain reaction”. Explain what is meant by the term “chain reaction”. ...
... Nuclear Radiation Ex4 1. In the reactor of a nuclear power station, neutrons split uranium nuclei to produce heat in what is known as a “chain reaction”. Explain what is meant by the term “chain reaction”. ...
1 - shawnschmitt
... for comparison f. Hypothesis- a possible explaination for observations, a testable idea g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. ...
... for comparison f. Hypothesis- a possible explaination for observations, a testable idea g. Mole- the amount of particles in 12g of Carbon-12, also, the amount of substance having 6.022x1023 of any kind of particle h. half-life- the amount of time required for ½ of the mass of an isotope to decay i. ...
Matter on Earth and in the universe is made of atoms that have
... located on the periodic table? ______________. The universe is composed mostly of __________ (light /heavy) elements. Why? The periodic table is ordered by atomic number or the number of protons. Increasing the number of protons and neutrons found in an atom increases its mass. Light elements are fo ...
... located on the periodic table? ______________. The universe is composed mostly of __________ (light /heavy) elements. Why? The periodic table is ordered by atomic number or the number of protons. Increasing the number of protons and neutrons found in an atom increases its mass. Light elements are fo ...
ps-5-1-and-5-2-ws
... 16.The average mass of all the isotopes of an element is called the ___________________________ 17.The modern periodic table is arranged in order of increasing _______________________________ 18. Information found on the periodic table for each element includes its atomic number, , name, and atomic ...
... 16.The average mass of all the isotopes of an element is called the ___________________________ 17.The modern periodic table is arranged in order of increasing _______________________________ 18. Information found on the periodic table for each element includes its atomic number, , name, and atomic ...
希臘 - 中正大學化生系
... corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule ...
... corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule ...
Protons neutrons electrons Charge Positive neutral negative Mass
... Atomic mass = weighted average of the naturally occurring isotopes • Atomic mass IS NOT THE SAME AS mass number!!! • Mass numbers are whole numbers, sum of protons and neutrons • Atomic numbers are averages so can be decimals and are found on the periodic table underneath element symbol • Atomic ...
... Atomic mass = weighted average of the naturally occurring isotopes • Atomic mass IS NOT THE SAME AS mass number!!! • Mass numbers are whole numbers, sum of protons and neutrons • Atomic numbers are averages so can be decimals and are found on the periodic table underneath element symbol • Atomic ...
The Atom
... Believed you never end up with a single particle EVER! Four Elements: earth, water, air, fire ...
... Believed you never end up with a single particle EVER! Four Elements: earth, water, air, fire ...
Timeline Assignment
... Early Greek philosophers believed all matter was made up of four “elements” earth, air, water, and fire ...
... Early Greek philosophers believed all matter was made up of four “elements” earth, air, water, and fire ...
IPS Unit 8 – Periodic Table Review Worksheet
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, eleme ...
... 7. An element is matter that is composed of one type of (atom/quark). 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, eleme ...
The Particle Theory of Matter
... 2. All particles of one substance are the __________. Different substances are made up of _____________________. 3. The particles are always _______________. The more energy the particles have, the _______________________. 4. There are attractive forces between the particles. These forces are strong ...
... 2. All particles of one substance are the __________. Different substances are made up of _____________________. 3. The particles are always _______________. The more energy the particles have, the _______________________. 4. There are attractive forces between the particles. These forces are strong ...
Table showing examples of Complex ions with their bond
... Metals and Non-Metals Definitions and Periodic trends Electronegativity is the power of a chemically bonded atom to attract electrons to itself. Electronegativity decreases down the group but increase across a period due increased distance between the valence electron and the nucleus i.e., greater a ...
... Metals and Non-Metals Definitions and Periodic trends Electronegativity is the power of a chemically bonded atom to attract electrons to itself. Electronegativity decreases down the group but increase across a period due increased distance between the valence electron and the nucleus i.e., greater a ...
Atomic Structure
... • Electrons closer to the nucleus have the lowest kinetic energy because of attractive forces between the electrons and protons. ...
... • Electrons closer to the nucleus have the lowest kinetic energy because of attractive forces between the electrons and protons. ...
Exam Review
... _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen ...
... _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen ...
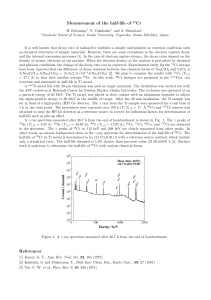
Measurement of the half-life of
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
... density of atomic electrons at the nucleus. When the electron density at the nucleus is perturbed by chemical and physical conditions, the change of the decay rate can be expected. Experimental study for the 51 Cr isotope have been reported that the difference of decay constant between two chemical f ...
CW07 Electron Structure of the Atom Click HERE for Puzzle Click
... A rule stating that a maximum of two electrons must have opposite spin is called ___ rule (7.3) Prefix multiplier for 10-12 (MT1.3) The principle energy levels correspond to the ___ numbers of the periodic table (7.3) Photon energy is ___ proportional to the frequency (7.1) A measure of the size of ...
... A rule stating that a maximum of two electrons must have opposite spin is called ___ rule (7.3) Prefix multiplier for 10-12 (MT1.3) The principle energy levels correspond to the ___ numbers of the periodic table (7.3) Photon energy is ___ proportional to the frequency (7.1) A measure of the size of ...
投影片 - 中正大學化生系
... corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule ...
... corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule ...
Review Outline for Atomic Structure Test
... _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen ...
... _P,As,Sb,Bi__8. Name of another element in the same family with Nitrogen _Li,Be,B,C,O,F,Ne____9. Name of another element in the same period with Nitrogen ...