ElementsPeriodicTable Notes
... An atom is composed of positively charged protons, neutral neutrons, and negatively charged electrons. Protons and neutrons are about equal in mass. An electron has about 1/2,000 the mass of a proton or neutron. ...
... An atom is composed of positively charged protons, neutral neutrons, and negatively charged electrons. Protons and neutrons are about equal in mass. An electron has about 1/2,000 the mass of a proton or neutron. ...
Tutorial 1
... 1. Use the second member of each group from Group 1A to Group 7A to show that the number of valance electrons on an atom of the element is the same as its group number. 2. Use Lewis dot symbol to show the formation of aluminum oxide (Al 2O3) 3. Explain what an ionic bond is? And name five metals and ...
... 1. Use the second member of each group from Group 1A to Group 7A to show that the number of valance electrons on an atom of the element is the same as its group number. 2. Use Lewis dot symbol to show the formation of aluminum oxide (Al 2O3) 3. Explain what an ionic bond is? And name five metals and ...
Atoms Vs. Molecules
... Isotopes (atoms with different numbers of protons and neutrons) can become unstable and are called radioactive. They can be used to identify certain chemical reactions, or for nuclear development. ...
... Isotopes (atoms with different numbers of protons and neutrons) can become unstable and are called radioactive. They can be used to identify certain chemical reactions, or for nuclear development. ...
TFSD Unwrapped Standard 3rd Math Algebra sample
... Skills: Be able to do (Verb Phrases) Explain how the scientists discovered the parts of the atom Diagram the electron configuration Identify the parts of the atom and where they are located Describe how waves and particles relate to electrons Differentiate between atoms, ions, and isotopes ...
... Skills: Be able to do (Verb Phrases) Explain how the scientists discovered the parts of the atom Diagram the electron configuration Identify the parts of the atom and where they are located Describe how waves and particles relate to electrons Differentiate between atoms, ions, and isotopes ...
Name January 5, 2017 Period Bio-Chem Unit 2 Review (Chapters
... 1. a compound is a pure substance consisting of two or more different elements in a fixed ratio. 2. Law of Definite Proportions states: different samples of any compound contain the same element in the same proportion by mass. Example: water is always found to have definite proportion 88.9% oxygen a ...
... 1. a compound is a pure substance consisting of two or more different elements in a fixed ratio. 2. Law of Definite Proportions states: different samples of any compound contain the same element in the same proportion by mass. Example: water is always found to have definite proportion 88.9% oxygen a ...
Grade 11 Chemistry Exam Review
... Element X consists of 30.00% of an isotope with mass 24.02 u and 70.00% of an isotope with mass 26.10 u. The average atomic mass of X is a) 24.64 u. b) 25.06 u. c) 25.48 u. d) 50.12 u ...
... Element X consists of 30.00% of an isotope with mass 24.02 u and 70.00% of an isotope with mass 26.10 u. The average atomic mass of X is a) 24.64 u. b) 25.06 u. c) 25.48 u. d) 50.12 u ...
Isotope Worksheet
... In other words, isotopes have the same number of protons but a different number of neutrons. Since any atom having 9 protons (Z = 9) must be an atom of fluorine, we can omit the Z-value and just use the symbol F for many purposes, i.e., we can write 19F instead of 19F. ...
... In other words, isotopes have the same number of protons but a different number of neutrons. Since any atom having 9 protons (Z = 9) must be an atom of fluorine, we can omit the Z-value and just use the symbol F for many purposes, i.e., we can write 19F instead of 19F. ...
Isotope Worksheet
... atom having 6 protons will be a "carbon" atom. If we were to add an extra proton to the nucleus, we would have an entirely different element. For example, ! ...
... atom having 6 protons will be a "carbon" atom. If we were to add an extra proton to the nucleus, we would have an entirely different element. For example, ! ...
Measuring the Atom
... If we look on the periodic table we will see that atomic mass of Carbon is 12.01u But didn’t we just say that Carbon was the standard at 12u???? The standard was Carbon-12 not Carbon (which is made up of all the isotopes: 12, 13, and 14) ...
... If we look on the periodic table we will see that atomic mass of Carbon is 12.01u But didn’t we just say that Carbon was the standard at 12u???? The standard was Carbon-12 not Carbon (which is made up of all the isotopes: 12, 13, and 14) ...
CHEMISTRY FALL FINAL PRACTICE 2016
... Describe each of the following in terms of valence electrons, block on periodic table, and outer electron configuration: alkali family, alkaline-earth metals, halogens and the noble gases by completing the table: ...
... Describe each of the following in terms of valence electrons, block on periodic table, and outer electron configuration: alkali family, alkaline-earth metals, halogens and the noble gases by completing the table: ...
Chapter 3 - Vocabulary and Notes
... A. Substance – Matter that has the same composition and properties throughout B. Compound – Substance whose smallest unit is made up of more than one element 1. Chemical Formula – tells which elements make up a compound as well as how many atoms of each element are present a. The subscript number te ...
... A. Substance – Matter that has the same composition and properties throughout B. Compound – Substance whose smallest unit is made up of more than one element 1. Chemical Formula – tells which elements make up a compound as well as how many atoms of each element are present a. The subscript number te ...
Chapter 4.1 and 4.2 - science-b
... In a chemical reaction, one substance changes to another by reorganizing the way the atoms are attached to each other ...
... In a chemical reaction, one substance changes to another by reorganizing the way the atoms are attached to each other ...
ATOMIC THEORY SCIENTISTS
... through indicating that atoms are mostly empty space. Some were shot directly backwards because they were repelled by the + nucleus. Others were slightly deflected because they came close to the repellent positive nucleus. 5. Neils Bohr (Plantary Model in 1913) said that the atom looked like a tiny ...
... through indicating that atoms are mostly empty space. Some were shot directly backwards because they were repelled by the + nucleus. Others were slightly deflected because they came close to the repellent positive nucleus. 5. Neils Bohr (Plantary Model in 1913) said that the atom looked like a tiny ...
AP Chemistry 2013 Semester 1 Final Exam Review Problems
... 12. An electron microscope employs a beam of electrons to obtain an image of an object. What energy must be imparted to each electron of the beam to obtain a wavelength of 10.0pm? Using the formula KE = ½mv2, calculate the energy in electron volts (eV) (1eV = 1.602 x 10-19J) 13. What is the differen ...
... 12. An electron microscope employs a beam of electrons to obtain an image of an object. What energy must be imparted to each electron of the beam to obtain a wavelength of 10.0pm? Using the formula KE = ½mv2, calculate the energy in electron volts (eV) (1eV = 1.602 x 10-19J) 13. What is the differen ...
ATOMIC THEORY OF MATTER
... which involve electron interactions. The electrons act as the “glue” between atoms. – If electrons are shared between two atoms, the bond is a covalent bond. I.e., the bond between two non-metal atoms. – If electrons are transferred to produce ions, the bond is ionic. • Ions are charged particles wh ...
... which involve electron interactions. The electrons act as the “glue” between atoms. – If electrons are shared between two atoms, the bond is a covalent bond. I.e., the bond between two non-metal atoms. – If electrons are transferred to produce ions, the bond is ionic. • Ions are charged particles wh ...
Chemistry 11 – Course Outcomes
... compounds occurs State octet rule Give an electron dot diagram (Lewis dot diagram) of atoms forming ions and ionic bonding (3 equations) Use model for the structure of an ionic compound to explain its properties Define molecular (covalent bonding) and give structural formula of molecular compounds. ...
... compounds occurs State octet rule Give an electron dot diagram (Lewis dot diagram) of atoms forming ions and ionic bonding (3 equations) Use model for the structure of an ionic compound to explain its properties Define molecular (covalent bonding) and give structural formula of molecular compounds. ...
atoms
... Rutherford and the Nucleus: Gold Foil Experiment A few particles deflected strongly Some bounced back!! Neutrons (no charge): located in center of atom Protons (+): positively charged particles inside the ...
... Rutherford and the Nucleus: Gold Foil Experiment A few particles deflected strongly Some bounced back!! Neutrons (no charge): located in center of atom Protons (+): positively charged particles inside the ...
Chemistry Study Guide What is matter made of? Matter is anything
... Elements are unique, pure substances. Elements and the Periodic Table Elements are arranged in order of their atomic number. The atomic number of an element is the number of protons in the nucleus of an atom of that element. Every element has its own atomic number. The periodic table has horizontal ...
... Elements are unique, pure substances. Elements and the Periodic Table Elements are arranged in order of their atomic number. The atomic number of an element is the number of protons in the nucleus of an atom of that element. Every element has its own atomic number. The periodic table has horizontal ...
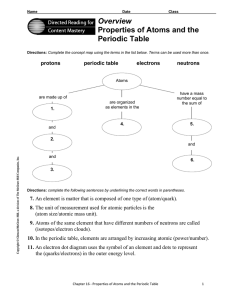
Overview Properties of Atoms and the Periodic Table
... Directions: Use the terms below to complete the following paragraphs about atoms, atomic mass, and isotopes. Terms may be used more than once. ...
... Directions: Use the terms below to complete the following paragraphs about atoms, atomic mass, and isotopes. Terms may be used more than once. ...
Atomic history - Kenton County Schools
... Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 204 ...
... Dorin, Demmin, Gabel, Chemistry The Study of Matter , 3rd Edition, 1990, page 204 ...
PowerPoint_Atomic Structure
... suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ...
... suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ...
Atomictheory
... Atomic Theory • All elements are composed of atoms that cannot be divided. • All atoms of the same element are exactly alike and have the same mass. Atoms of different elements are different and have different masses. • An atom of one element cannot be changed into an atom of different elements. At ...
... Atomic Theory • All elements are composed of atoms that cannot be divided. • All atoms of the same element are exactly alike and have the same mass. Atoms of different elements are different and have different masses. • An atom of one element cannot be changed into an atom of different elements. At ...