Double Displacement Reactions
... A Reaction That Forms a Gas Sometimes, the production of a gas, rather than a precipitate, indicates that a double displacement reaction has occurred. Many of these double displacement reactions are, in fact, two reactions that occur in rapid succession. A double displacement occurs, but then one o ...
... A Reaction That Forms a Gas Sometimes, the production of a gas, rather than a precipitate, indicates that a double displacement reaction has occurred. Many of these double displacement reactions are, in fact, two reactions that occur in rapid succession. A double displacement occurs, but then one o ...
Personal Tutoring Help on Questions and Problems
... of it has a mass of 152 g. 3.26 How many molecules of ethane (C2H6) are present in 0.334 g of C2H6? 3.27 Calculate the number of C, H, and O atoms in 1.50 g of glucose (C6H12O6), a sugar. 3.28 Urea [(NH2)2CO] is used for fertilizer and many other things. Calculate the number of N, C, O, and H atoms ...
... of it has a mass of 152 g. 3.26 How many molecules of ethane (C2H6) are present in 0.334 g of C2H6? 3.27 Calculate the number of C, H, and O atoms in 1.50 g of glucose (C6H12O6), a sugar. 3.28 Urea [(NH2)2CO] is used for fertilizer and many other things. Calculate the number of N, C, O, and H atoms ...
In order to grasp the concept of molar mass calculations it is
... you might find in the chemistry laboratory can range between 18 grams/mole for compounds like water to hundreds of grams per mole for more complex chemical compounds. The lightest possible chemical that one can have under normal conditions is hydrogen gas, or H2. There is no limit to how heavy a che ...
... you might find in the chemistry laboratory can range between 18 grams/mole for compounds like water to hundreds of grams per mole for more complex chemical compounds. The lightest possible chemical that one can have under normal conditions is hydrogen gas, or H2. There is no limit to how heavy a che ...
CHAPTER 21 NONMETALLIC ELEMENTS AND THEIR COMPOUNDS
... Eventually, the sodium hydrogen carbonate precipitates (the water solvent evaporates since NaHCO3 is not hygroscopic). Thus, most of the white solid is NaHCO3 plus some Na2CO3. ...
... Eventually, the sodium hydrogen carbonate precipitates (the water solvent evaporates since NaHCO3 is not hygroscopic). Thus, most of the white solid is NaHCO3 plus some Na2CO3. ...
Chapter 3
... cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula. – Nitrogen and oxygen form all of the following unique molecular compounds: NO, NO2, N2O, N2O3, N2O4, and N2O5. © 2014 Pears ...
... cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula. – Nitrogen and oxygen form all of the following unique molecular compounds: NO, NO2, N2O, N2O3, N2O4, and N2O5. © 2014 Pears ...
Ionic Compounds
... cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula. – Nitrogen and oxygen form all of the following unique molecular compounds: NO, NO2, N2O, N2O3, N2O4, and N2O5. © 2014 Pears ...
... cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula. – Nitrogen and oxygen form all of the following unique molecular compounds: NO, NO2, N2O, N2O3, N2O4, and N2O5. © 2014 Pears ...
compounds - Belle Vernon Area
... cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula. – Nitrogen and oxygen form all of the following unique molecular compounds: NO, NO2, N2O, N2O3, N2O4, and N2O5. © 2014 Pears ...
... cannot readily be determined from its constituent elements because the same combination of elements may form many different molecular compounds, each with a different formula. – Nitrogen and oxygen form all of the following unique molecular compounds: NO, NO2, N2O, N2O3, N2O4, and N2O5. © 2014 Pears ...
FREE Sample Here - We can offer most test bank and
... 7. Which of these is the best definition of a scientific theory? a. A prediction based on a limited number of observations. b. A method of explaining observations that appears contradictory. c. A broadly applicable generalization with virtually no exceptions. d. A method for approaching problems tha ...
... 7. Which of these is the best definition of a scientific theory? a. A prediction based on a limited number of observations. b. A method of explaining observations that appears contradictory. c. A broadly applicable generalization with virtually no exceptions. d. A method for approaching problems tha ...
Measuring Rates
... The analysis of the kinetic stability of biomolecules has been crucial in the evaluation of different theories about the origin of life. For example, chemical species needed for the synthesis of amino acids, such as CH4 and NH3, are abundant in hydrothermal vent regions with temperatures between 60 ...
... The analysis of the kinetic stability of biomolecules has been crucial in the evaluation of different theories about the origin of life. For example, chemical species needed for the synthesis of amino acids, such as CH4 and NH3, are abundant in hydrothermal vent regions with temperatures between 60 ...
ppt - Wits Structural Chemistry
... If the chemical formula of a substance is the same as its molecular formula, then the formula weight is also called the molecular weight. Molecular weight (Mr or MW) is the mass of a collection of atoms represented by a chemical formula for a molecule. What is the molecular formula for glucose? MW( ...
... If the chemical formula of a substance is the same as its molecular formula, then the formula weight is also called the molecular weight. Molecular weight (Mr or MW) is the mass of a collection of atoms represented by a chemical formula for a molecule. What is the molecular formula for glucose? MW( ...
Chapter 7 - NordoniaHonorsChemistry
... reaction. A complete ionic equation is a chemical equation showing all of the species as they are actually present in solution. A net ionic equation is an equation showing only the species that actually participate in the reaction. ...
... reaction. A complete ionic equation is a chemical equation showing all of the species as they are actually present in solution. A net ionic equation is an equation showing only the species that actually participate in the reaction. ...
Knowledge Check (Answer Key)
... components of matter that retain the identifying properties of an element. Each element is made up of atoms identified by a unique combination of subatomic particles making up their nuclei and orbiting fields. When an atom’s subatomic particle configuration is changed, the atom’s elemental identific ...
... components of matter that retain the identifying properties of an element. Each element is made up of atoms identified by a unique combination of subatomic particles making up their nuclei and orbiting fields. When an atom’s subatomic particle configuration is changed, the atom’s elemental identific ...
Evidence for the Predominance of Condensed Phase Reaction in
... toward the results. To determine the reaction rate from our experiment, we consider the oxidation of one carbon nanoparticle within the time scale of the experiment. Assuming a 50 nm carbon particle (mass of ∼1.3 × 10−16 g) where the reaction occurs at the surface in a time of ∼0.9 ms (average rise ...
... toward the results. To determine the reaction rate from our experiment, we consider the oxidation of one carbon nanoparticle within the time scale of the experiment. Assuming a 50 nm carbon particle (mass of ∼1.3 × 10−16 g) where the reaction occurs at the surface in a time of ∼0.9 ms (average rise ...
Stoichiometry: Calculations with Chemical Formulas and
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
Objective: To state the assumptions of Dalton`s Atomic Theory
... Objective: To state the assumptions of Dalton's Atomic Theory. Explain the contributions of Dalton, Thompson, Rutherford to our understanding of the atom. Democritus 400 BC: atomos "indivisible" Dalton (early 1800's) Atomic Theory ...
... Objective: To state the assumptions of Dalton's Atomic Theory. Explain the contributions of Dalton, Thompson, Rutherford to our understanding of the atom. Democritus 400 BC: atomos "indivisible" Dalton (early 1800's) Atomic Theory ...
AS Specification pdf | AS/A level
... This section outlines the knowledge, understanding and skills to be developed by learners studying AS Chemistry. Learners should be prepared to apply the knowledge, understanding and skills specified in a range of theoretical, practical, industrial and environmental contexts. It is a requirement of ...
... This section outlines the knowledge, understanding and skills to be developed by learners studying AS Chemistry. Learners should be prepared to apply the knowledge, understanding and skills specified in a range of theoretical, practical, industrial and environmental contexts. It is a requirement of ...
Atomic Structure Chapter Seven
... Millikan’s Oil Drop Experiment • George Stoney: names the cathode-ray particle the electron. • Robert Millikan: determines a value for the electron’s charge: e = –1.602 ×10–19 C Charged droplet can move either up or down, depending on the charge on the plates. ...
... Millikan’s Oil Drop Experiment • George Stoney: names the cathode-ray particle the electron. • Robert Millikan: determines a value for the electron’s charge: e = –1.602 ×10–19 C Charged droplet can move either up or down, depending on the charge on the plates. ...
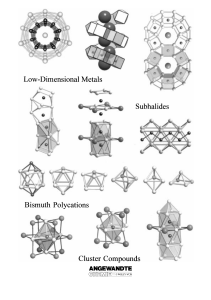
From the Metal to the Molecule
... Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research m ...
... Such subvalent compounds are not only known for electron-deficient s-, d-, and f-metals, but also for p-(semi-) metals albeit with far fewer examples. For example, recently significant progress has been made especially in research using the elements indium,[6] tin,[7] and bismuth.[8] This research m ...
CHEM181H1_06_2013_Y_P1
... This paper consists of 16 pages including the cover page, periodic table and two data sheets. Please ensure that you have them all. The use of non-programmable electronic calculators is permitted. ANSWER ALL QUESTIONS DIRECTLY ON THE PAPER AND WHERE NECESSARY OVER THE PAGE. Examiner ...
... This paper consists of 16 pages including the cover page, periodic table and two data sheets. Please ensure that you have them all. The use of non-programmable electronic calculators is permitted. ANSWER ALL QUESTIONS DIRECTLY ON THE PAPER AND WHERE NECESSARY OVER THE PAGE. Examiner ...
Chapter 10
... Strategy Arrange the equations that are provided so that they will sum to the desired equation. This may require reversing or multiplying one or more of the equations. For any such change, the corresponding change must also be made to the ΔH rxn ° value. The desired equation, corresponding to the st ...
... Strategy Arrange the equations that are provided so that they will sum to the desired equation. This may require reversing or multiplying one or more of the equations. For any such change, the corresponding change must also be made to the ΔH rxn ° value. The desired equation, corresponding to the st ...
SOME BASIC CONCEPTS OF CHEMISTRY NOTES
... 50mL) bear a simple ratio of 2:1 AVOGADRO LAW: Equal volumes of all gases contain equal number of molecules under the same conditions of temperature and pressure. DALTON’S ATOMIC THEORY: In 1808, Dalton proposed hi atomic theory. According to this theory, ...
... 50mL) bear a simple ratio of 2:1 AVOGADRO LAW: Equal volumes of all gases contain equal number of molecules under the same conditions of temperature and pressure. DALTON’S ATOMIC THEORY: In 1808, Dalton proposed hi atomic theory. According to this theory, ...
3 - LPS
... Identification. State whether each of the following is a physical or chemical change or property by writing an ”A” if it is a physical change, “B” if it is a physical property, “C” if it is a chemical change, or “D” if it is a chemical property. ...
... Identification. State whether each of the following is a physical or chemical change or property by writing an ”A” if it is a physical change, “B” if it is a physical property, “C” if it is a chemical change, or “D” if it is a chemical property. ...