Unit 5 2 Thermodynamics Enthalpy
... * any element is zero!!! i) due to the fact that there is no formation reaction needed when the element is already in its standard state. e.g.) the ∆H°f for graphite, H2(g), O2(g) and all other elements = 0 kJ/mol ...
... * any element is zero!!! i) due to the fact that there is no formation reaction needed when the element is already in its standard state. e.g.) the ∆H°f for graphite, H2(g), O2(g) and all other elements = 0 kJ/mol ...
AP Stoichiometry
... In one process, 124 g of Al are reacted with 601 g of Fe2O3 2Al + Fe2O3 Al2O3 + 2Fe Calculate the mass of Al2O3 formed. g Al ...
... In one process, 124 g of Al are reacted with 601 g of Fe2O3 2Al + Fe2O3 Al2O3 + 2Fe Calculate the mass of Al2O3 formed. g Al ...
Chapter 2 Atoms, molecules and ions
... of another. But we don't consider processes that affect the nucleus to be chemical processes. The postulate is still useful. A slightly more restrictive wording is "Atoms cannot be created, destroyed, or transformed into other atoms in a chemical change". ...
... of another. But we don't consider processes that affect the nucleus to be chemical processes. The postulate is still useful. A slightly more restrictive wording is "Atoms cannot be created, destroyed, or transformed into other atoms in a chemical change". ...
Chapter 4.1 Slides
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
... Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. ...
Unit 8: Reactions
... 8. Oxidation: The loss of electron(s), causing the oxidation number of a species to become more positive. 9. Precipitate: An insoluble solid that is formed in either a doublereplacement reaction or as an excess solute added to a saturated solution. 10. Product: The substances that are formed by a ch ...
... 8. Oxidation: The loss of electron(s), causing the oxidation number of a species to become more positive. 9. Precipitate: An insoluble solid that is formed in either a doublereplacement reaction or as an excess solute added to a saturated solution. 10. Product: The substances that are formed by a ch ...
thermodynamics
... do not. Explain whether the following properties are extensive or intensive. Mass, internal energy, pressure, heat capacity, molar heat capacity, density, mole fraction, specific heat, temperature and molarity. 60. The lattice enthalpy of an ionic compound is the enthalpy when one mole of an ionic c ...
... do not. Explain whether the following properties are extensive or intensive. Mass, internal energy, pressure, heat capacity, molar heat capacity, density, mole fraction, specific heat, temperature and molarity. 60. The lattice enthalpy of an ionic compound is the enthalpy when one mole of an ionic c ...
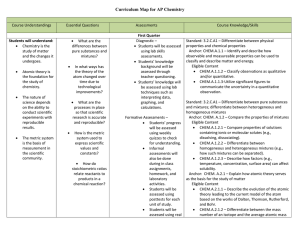
AP Chemistry Curriculum Map - Belle Vernon Area School District
... orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals). Anchor: CHEM.A.2.3 – Explain how periodic trends in the properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.1 – Explain how the periodicity of chemical pro ...
... orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals). Anchor: CHEM.A.2.3 – Explain how periodic trends in the properties of atoms allow for the prediction of physical and chemical properties. Eligible Content CHEM.A.2.3.1 – Explain how the periodicity of chemical pro ...
Stoichiometry - HCC Learning Web
... Step 1: Write the balanced chemical equation for the reaction. Step 2: Calculate the moles of "given" substance. If more than one reactant amount is given, calculate the moles of each to determine which is the limiting reactant. Step 3: Calculate the moles of "desired" substance from your answer in ...
... Step 1: Write the balanced chemical equation for the reaction. Step 2: Calculate the moles of "given" substance. If more than one reactant amount is given, calculate the moles of each to determine which is the limiting reactant. Step 3: Calculate the moles of "desired" substance from your answer in ...
1st-Year-ch-wise-test
... (1) A complete chemical characterization of a compound include (a) quantitative analysis (b) qualitative analysis (c) analytical chemistry (d) both a & b (2) Insoluble particles in a liquid are removed by (a) sublimation (b) solvent extraction (c) crystallization (d) filtration (3) To obtain medium ...
... (1) A complete chemical characterization of a compound include (a) quantitative analysis (b) qualitative analysis (c) analytical chemistry (d) both a & b (2) Insoluble particles in a liquid are removed by (a) sublimation (b) solvent extraction (c) crystallization (d) filtration (3) To obtain medium ...
Chapter 3: Elements, Compounds and the Periodic Table
... Atomic number (Z) Number of protons that atom has in nucleus Unique to each type of element Element is substance whose atoms all contain identical number of protons ...
... Atomic number (Z) Number of protons that atom has in nucleus Unique to each type of element Element is substance whose atoms all contain identical number of protons ...
SELECTED ANSWERS
... 33. When solid lithium iodide is added to water, all of the ions at the surface of the solid can be viewed as vibrating back and forth between moving out into the water and returning to the solid surface. Sometimes when an ion vibrates out into the water, a water molecule collides with it, helping t ...
... 33. When solid lithium iodide is added to water, all of the ions at the surface of the solid can be viewed as vibrating back and forth between moving out into the water and returning to the solid surface. Sometimes when an ion vibrates out into the water, a water molecule collides with it, helping t ...
Chapter 3
... atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it is huge number is not?!!!! It is clear that we can not weight a single atom, but as ...
... atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it is huge number is not?!!!! It is clear that we can not weight a single atom, but as ...
Sample pages 2 PDF
... that is, the graph of ln[A] as a function of t gives a straight line whose intercept is ln [A]0 and whose slope is equal to −k. If we now consider the time interval t = t1/2 such that [A] is half its initial value, [A] = [A]0/2, then substitution of these equalities in (2.13) leads to t1=2 ¼ ln2=k ...
... that is, the graph of ln[A] as a function of t gives a straight line whose intercept is ln [A]0 and whose slope is equal to −k. If we now consider the time interval t = t1/2 such that [A] is half its initial value, [A] = [A]0/2, then substitution of these equalities in (2.13) leads to t1=2 ¼ ln2=k ...
Full text
... exert any influence on the energy levels. An obvious consequence is the fact that excited states, where two electrons are in singly occupied orbitals, will have the same energy whether they are singlet or triplet. (2) Since every electron has an MO which is computed as if other electrons were not pr ...
... exert any influence on the energy levels. An obvious consequence is the fact that excited states, where two electrons are in singly occupied orbitals, will have the same energy whether they are singlet or triplet. (2) Since every electron has an MO which is computed as if other electrons were not pr ...
Photocatalysis on TiOn Surfaces: Principles, Mechanisms, and
... dipole in a ground state quencher molecule. This coupling process does not require effective orbital overlap between the two interacting centers and can operate over a distance range from less than 10 A to as large as 100 A.10 There exists a vast body of literature dealing with the electron transfer ...
... dipole in a ground state quencher molecule. This coupling process does not require effective orbital overlap between the two interacting centers and can operate over a distance range from less than 10 A to as large as 100 A.10 There exists a vast body of literature dealing with the electron transfer ...
24. The following reaction is at equilibrium
... 22. The following statements refer to a mixture (reaction quotient Q) that is prepared and then allowed to come to equilibrium. Which statement is NOT CORRECT? (A) A reaction will proceed to make the value of Q approach that of K. (B) If Q = K there is no change. (C) If Q > K, the reaction goes to ...
... 22. The following statements refer to a mixture (reaction quotient Q) that is prepared and then allowed to come to equilibrium. Which statement is NOT CORRECT? (A) A reaction will proceed to make the value of Q approach that of K. (B) If Q = K there is no change. (C) If Q > K, the reaction goes to ...
Chemistry, Biology
... question(s) will, likewise, be common to the respective papers. This Paper will be set at the same time for all three syllabuses, 5076, 5077, and 5078. The use of reference material, other than the Chemistry Practical Notes is not permitted. In one or both questions, candidates will be expected to s ...
... question(s) will, likewise, be common to the respective papers. This Paper will be set at the same time for all three syllabuses, 5076, 5077, and 5078. The use of reference material, other than the Chemistry Practical Notes is not permitted. In one or both questions, candidates will be expected to s ...
Physics, Chemistry
... question(s) will, likewise, be common to the respective papers. This Paper will be set at the same time for all three syllabuses, 5076, 5077, and 5078. The use of reference material, other than the Chemistry Practical Notes is not permitted. In one or both questions, candidates will be expected to s ...
... question(s) will, likewise, be common to the respective papers. This Paper will be set at the same time for all three syllabuses, 5076, 5077, and 5078. The use of reference material, other than the Chemistry Practical Notes is not permitted. In one or both questions, candidates will be expected to s ...