ChemQuest 1 Information: Qualitative vs. Quantitative Critical
... Books are made of matter. You are made of matter. “Matter” is a fancy word for the “stuff” of which all objects are made. Every day, matter is changed in different ways. For example, paper can be changed in many ways—it can be torn, folded, or burned. A chemical change is any alteration that changes ...
... Books are made of matter. You are made of matter. “Matter” is a fancy word for the “stuff” of which all objects are made. Every day, matter is changed in different ways. For example, paper can be changed in many ways—it can be torn, folded, or burned. A chemical change is any alteration that changes ...
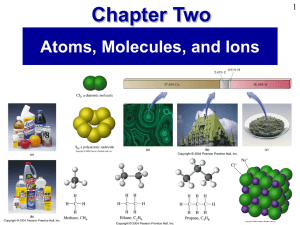
Prentice Hall Ch 02 Atoms Molecules Ions
... front cover to determine which of the three is the most abundant. Can you determine which is the second most abundant? Explain. ...
... front cover to determine which of the three is the most abundant. Can you determine which is the second most abundant? Explain. ...
Diversity-oriented synthesis - David Spring
... TOS aims to synthesize a molecule at a discrete point in chemical space, DOS aims to cover as diffuse an area as possible.12 The distinction between DOS and combinatorial chemistry is less clear cut. We would therefore like to suggest that DOS is a more “evolved” version of combinatorial chemistry. ...
... TOS aims to synthesize a molecule at a discrete point in chemical space, DOS aims to cover as diffuse an area as possible.12 The distinction between DOS and combinatorial chemistry is less clear cut. We would therefore like to suggest that DOS is a more “evolved” version of combinatorial chemistry. ...
Document
... 1 mole of O2 can at most produce 2 moles of water (theoretical yield is 36 g since molar mass of water is about 18 g/mol. So %yield = (9g/36g)x100=25% a) 400% ...
... 1 mole of O2 can at most produce 2 moles of water (theoretical yield is 36 g since molar mass of water is about 18 g/mol. So %yield = (9g/36g)x100=25% a) 400% ...
Thermodynamics and Equilibrium
... spontaneously because doing so lowers its energy, but it does not move back up the hill spontaneously because an input of energy is required to do so. Thus, it would be tempting to conclude that processes should be spontaneous if they are exothermic. However, there are many examples of spontaneous e ...
... spontaneously because doing so lowers its energy, but it does not move back up the hill spontaneously because an input of energy is required to do so. Thus, it would be tempting to conclude that processes should be spontaneous if they are exothermic. However, there are many examples of spontaneous e ...
POGIL - Basic Skills Supplement - The Mole-1
... A mole is a way to quantify atoms and molecules. The mole is a critical component when working with chemical reactions. The purpose of the mole is to normalize quantities of atoms and molecules when working with them in chemical reactions. In a molecule of water (H2O), for example, there are two hy ...
... A mole is a way to quantify atoms and molecules. The mole is a critical component when working with chemical reactions. The purpose of the mole is to normalize quantities of atoms and molecules when working with them in chemical reactions. In a molecule of water (H2O), for example, there are two hy ...
File
... the study of quantitative relationships between amounts of ______________________________________________________________ reactants used and products formed by a chemical reaction ______________________________________________________________ ...
... the study of quantitative relationships between amounts of ______________________________________________________________ reactants used and products formed by a chemical reaction ______________________________________________________________ ...
Unit F325 - Equilibria, energetics and elements - High band
... numbers (–2 is actually ‘more’ than –4!). The candidate should have stated that the lattice enthalpy of MgO is less exothermic or less negative’. By using just ‘less’, this is a case is which ‘less means more’! . ...
... numbers (–2 is actually ‘more’ than –4!). The candidate should have stated that the lattice enthalpy of MgO is less exothermic or less negative’. By using just ‘less’, this is a case is which ‘less means more’! . ...
Chapter 19: Thermochemistry II: Entropy and free Energy
... given temperature, the entropies of gases are, in general, higher than liquids, which are higher than solids. Heavier or more complicated substances tend to have higher entropies than lighter or simpler substances because they have more ways to store thermal energy at ...
... given temperature, the entropies of gases are, in general, higher than liquids, which are higher than solids. Heavier or more complicated substances tend to have higher entropies than lighter or simpler substances because they have more ways to store thermal energy at ...
advanced placement chemistry workbook and note set
... carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You can see in the figure above that there are three different isotopes of carbon atoms present in the lump of coal. The atoms do not differ in atomic number (because a ...
... carbon-13 and the isotope on the right is carbon-14. This system of naming isotopes helps distinguish between the varying Figure 4. You can see in the figure above that there are three different isotopes of carbon atoms present in the lump of coal. The atoms do not differ in atomic number (because a ...
Chemical Quantities: Stoichiometry and the Mole
... You should also note that, as I mentioned in my “steps” in doing stoichiometric calculations, you KNOW before you do any number crunching on your calculator whether you are heading the right way in solving the problem because the units cancel. Look back at the examples and notice the cancelled units ...
... You should also note that, as I mentioned in my “steps” in doing stoichiometric calculations, you KNOW before you do any number crunching on your calculator whether you are heading the right way in solving the problem because the units cancel. Look back at the examples and notice the cancelled units ...
Tests
... _____ Microscopic refers to the small particles that make up all matter. _____ Observing the rusting of iron is a microscopic process. _____ Chemistry is the study of planetary orbits. _____ The alchemists were never successful in their attempts to make gold. _____ The elixir of life was supposed to ...
... _____ Microscopic refers to the small particles that make up all matter. _____ Observing the rusting of iron is a microscopic process. _____ Chemistry is the study of planetary orbits. _____ The alchemists were never successful in their attempts to make gold. _____ The elixir of life was supposed to ...
Name:
... changes only slightly and therefore does not offset the increase in size due to the increase in energy levels. Atomic radius decreases as you go left to right across a period in the periodic table. The valence electrons are found in orbitals of the same energy level. At the same time, the effective ...
... changes only slightly and therefore does not offset the increase in size due to the increase in energy levels. Atomic radius decreases as you go left to right across a period in the periodic table. The valence electrons are found in orbitals of the same energy level. At the same time, the effective ...
CfE Advanced Higher Chemistry
... Electromagnetic radiation is a form of energy. Light, x-rays, radio signals and microwaves are all forms of electromagnetic radiation. Visible light is only a small part of the range of the electromagnetic spectrum. Figure 1.1: The electromagnetic spectrum ...
... Electromagnetic radiation is a form of energy. Light, x-rays, radio signals and microwaves are all forms of electromagnetic radiation. Visible light is only a small part of the range of the electromagnetic spectrum. Figure 1.1: The electromagnetic spectrum ...
chemistry - Ethiopian Ministry of Education
... 1.1.2 Major Fields of Chemistry The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated ...
... 1.1.2 Major Fields of Chemistry The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated ...
Molecular Orbitals - The Oakwood School
... – sigma bond ( bond): a bond formed when two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting the two atomic nuclei – pi bond ( bond): a covalent bond in which the bonding electrons are most likely to be found in sausage-shaped regions above and be ...
... – sigma bond ( bond): a bond formed when two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting the two atomic nuclei – pi bond ( bond): a covalent bond in which the bonding electrons are most likely to be found in sausage-shaped regions above and be ...
Chemistry 121: Topic 2 - From Atoms to Stoichiometry Topic 2
... Chemistry 121: Topic 2 - From Atoms to Stoichiometry Atomic Number, Mass Number, and Isotopes ¾ Atoms can be identified by the number of protons and neutrons they contain ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of pr ...
... Chemistry 121: Topic 2 - From Atoms to Stoichiometry Atomic Number, Mass Number, and Isotopes ¾ Atoms can be identified by the number of protons and neutrons they contain ¾ The atomic number (Z) is the number of protons in the nucleus of each atom of an element. ¾ In a neutral atom the number of pr ...
Dr. Spencer`s PPT
... Paris, and although Lavoisier's father wanted him to be a lawyer, Lavoisier was fascinated by science. From the beginning of his scientific career, Lavoisier recognized the importance of accurate measurements. He wrote the first modern chemistry (1789) textbook so that it is not surprising that Lavo ...
... Paris, and although Lavoisier's father wanted him to be a lawyer, Lavoisier was fascinated by science. From the beginning of his scientific career, Lavoisier recognized the importance of accurate measurements. He wrote the first modern chemistry (1789) textbook so that it is not surprising that Lavo ...
ХИМИЯ НА АНГЛИЙСКОМ ЯЗЫКЕ
... 2.6. Answer these questions for 0.25 mol of Al2O3. Find: a) the number of molecules it contains; b) the mole number of each kind of atom it contains; c) the total number of atoms it contains. 2.7. Answer these questions for 2.4x1024 SO3 molecules. Find: a) its mole number; b) the number of moles of ...
... 2.6. Answer these questions for 0.25 mol of Al2O3. Find: a) the number of molecules it contains; b) the mole number of each kind of atom it contains; c) the total number of atoms it contains. 2.7. Answer these questions for 2.4x1024 SO3 molecules. Find: a) its mole number; b) the number of moles of ...
Chapter 3 PPt
... Subatomic Particles, continued Rutherford Discovers the Nucleus, continued • Rutherford reasoned that only a very concentrated positive charge in a tiny space within the gold atom could possibly repel the fast-moving, alpha particles enough to reverse the alpha particles’ direction. • Rutherford als ...
... Subatomic Particles, continued Rutherford Discovers the Nucleus, continued • Rutherford reasoned that only a very concentrated positive charge in a tiny space within the gold atom could possibly repel the fast-moving, alpha particles enough to reverse the alpha particles’ direction. • Rutherford als ...
DOE Chemistry 1
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
... for use by DOE category A reactors. The subject areas, subject matter content, and level of detail of the Reactor Operator Fundamentals Manuals were determined from several sources. DOE Category A reactor training managers determined which materials should be included, and served as a primary refere ...
Personal Tutoring Help on Questions and Problems
... of it has a mass of 152 g. 3.26 How many molecules of ethane (C2H6) are present in 0.334 g of C2H6? 3.27 Calculate the number of C, H, and O atoms in 1.50 g of glucose (C6H12O6), a sugar. 3.28 Urea [(NH2)2CO] is used for fertilizer and many other things. Calculate the number of N, C, O, and H atoms ...
... of it has a mass of 152 g. 3.26 How many molecules of ethane (C2H6) are present in 0.334 g of C2H6? 3.27 Calculate the number of C, H, and O atoms in 1.50 g of glucose (C6H12O6), a sugar. 3.28 Urea [(NH2)2CO] is used for fertilizer and many other things. Calculate the number of N, C, O, and H atoms ...
In order to grasp the concept of molar mass calculations it is
... you might find in the chemistry laboratory can range between 18 grams/mole for compounds like water to hundreds of grams per mole for more complex chemical compounds. The lightest possible chemical that one can have under normal conditions is hydrogen gas, or H2. There is no limit to how heavy a che ...
... you might find in the chemistry laboratory can range between 18 grams/mole for compounds like water to hundreds of grams per mole for more complex chemical compounds. The lightest possible chemical that one can have under normal conditions is hydrogen gas, or H2. There is no limit to how heavy a che ...