Dalton Model Reading
... formulated by Antoine Lavoisier in 1789, which states that the total mass in a chemical reaction remains constant (that is, the reactants have the same mass as the products). The second was the law of definite proportions. First proven by the French chemist Joseph Louis Proust in 1799, this law stat ...
... formulated by Antoine Lavoisier in 1789, which states that the total mass in a chemical reaction remains constant (that is, the reactants have the same mass as the products). The second was the law of definite proportions. First proven by the French chemist Joseph Louis Proust in 1799, this law stat ...
chem1chapter3fromheisenberg
... Areas of high probability for electrons are orbitals • Each orbital can only have 2 electrons • What do the orbitals look like? ...
... Areas of high probability for electrons are orbitals • Each orbital can only have 2 electrons • What do the orbitals look like? ...
Atomic Theory Gallery Walk Stations
... the sun. All electrons have their energy levels – a certain distance from the nucleus. Each energy level can hold a certain number of electrons. Level 1 can hold 2 electrons Level 2– 8 electrons Level 3 – 18 electrons Level 4 – 32 electrons The energy of electrons goes up from Level 1 to other level ...
... the sun. All electrons have their energy levels – a certain distance from the nucleus. Each energy level can hold a certain number of electrons. Level 1 can hold 2 electrons Level 2– 8 electrons Level 3 – 18 electrons Level 4 – 32 electrons The energy of electrons goes up from Level 1 to other level ...
Chapter 3
... Electrons act like both particles and _____________. Thomson’s experiments demonstrated that electrons act like ____________ that have mass. In 1924, Louis de Broglie pointed out that the behavior of electrons according to Bohr’s model was similar to the behavior of _________________. De Broglie su ...
... Electrons act like both particles and _____________. Thomson’s experiments demonstrated that electrons act like ____________ that have mass. In 1924, Louis de Broglie pointed out that the behavior of electrons according to Bohr’s model was similar to the behavior of _________________. De Broglie su ...
Chemistry Semester Test Study Guide Chapters
... What state of matter has a definite volume and takes the shape of its container? Which state of matter takes both the shape and volume of its container? In a chemical reaction, what are the reactants and what are the products? If the total mass of the reactants in a chemical reaction is 60 g, what i ...
... What state of matter has a definite volume and takes the shape of its container? Which state of matter takes both the shape and volume of its container? In a chemical reaction, what are the reactants and what are the products? If the total mass of the reactants in a chemical reaction is 60 g, what i ...
Atom Democritus Dalton Thompson Rutherford Bohr Electron Cloud
... 2. Atoms of the same element are exactly alike 3. Compounds are formed by combining atoms 4. Chemical reactions are rearrangements of atoms ...
... 2. Atoms of the same element are exactly alike 3. Compounds are formed by combining atoms 4. Chemical reactions are rearrangements of atoms ...
An element`s properties depend on the structure of its atoms
... • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of pote ...
... • Energy is the capacity to cause change, perhaps by doing work. • Potential energy is the energy that matter has because of its location or structure, there are many kinds…not just gravitational PE! • The electrons of an atom differ in their amounts of potential energy • An electron’s state of pote ...
Atoms, Molecules, and Ions
... 2)-Compounds are composed of atoms of more than one element and in any compound the ratio of the number of atoms of any two elements present is an integer or simple fraction. 3)-A chemical reaction involves the separation, combination, or rearrangement of atoms, however atoms are not created or dest ...
... 2)-Compounds are composed of atoms of more than one element and in any compound the ratio of the number of atoms of any two elements present is an integer or simple fraction. 3)-A chemical reaction involves the separation, combination, or rearrangement of atoms, however atoms are not created or dest ...
Name Per ___ Reading Assignment – Chapter 4 pages 100
... Review for the test is page 121 and 122 Assessment. You need to be sure to go over this prior to our test over this material Words to know for this chapter Proton ___________________________________________________________________________ ground state. _______________________________________________ ...
... Review for the test is page 121 and 122 Assessment. You need to be sure to go over this prior to our test over this material Words to know for this chapter Proton ___________________________________________________________________________ ground state. _______________________________________________ ...
Properties of Matter Power Point
... Neutron- A sub-atomic particle in the nucleus of an atom. Neutrons are neutral, meaning that they have no charge, and a mass of 1 amu. Electron- A sub-atomic particle orbiting outside the nucleus of an atom. Electrons have a negative electrical charge and no mass. Atoms in their most stable state ha ...
... Neutron- A sub-atomic particle in the nucleus of an atom. Neutrons are neutral, meaning that they have no charge, and a mass of 1 amu. Electron- A sub-atomic particle orbiting outside the nucleus of an atom. Electrons have a negative electrical charge and no mass. Atoms in their most stable state ha ...
Name:______ Chemistry 114 First Hour Exam
... electrons in the first ð bond must be above and below the paper. The electrons in the next ð bond must be in a different plane, so they must be in the plane of the paper. The final ð bond must be above and below the paper, making the final hydrogens in the plane of the paper ...
... electrons in the first ð bond must be above and below the paper. The electrons in the next ð bond must be in a different plane, so they must be in the plane of the paper. The final ð bond must be above and below the paper, making the final hydrogens in the plane of the paper ...
Carbon Isotopes
... All atoms of the same element have the same number of protons. However, atoms may have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes of that element. Within a sample of oxygen, some atoms can have 8, 9 or 10 neutrons - these are the d ...
... All atoms of the same element have the same number of protons. However, atoms may have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes of that element. Within a sample of oxygen, some atoms can have 8, 9 or 10 neutrons - these are the d ...
Chemistry of Life
... Isotopes • An isotope is a variation of chemical element due to a different number of neutrons. • Identify isotopes by the atomic mass • Since the number of electrons remains the same the chemical properties remain the same • Radioactive isotopes have an unstable nuclei and will eventually break do ...
... Isotopes • An isotope is a variation of chemical element due to a different number of neutrons. • Identify isotopes by the atomic mass • Since the number of electrons remains the same the chemical properties remain the same • Radioactive isotopes have an unstable nuclei and will eventually break do ...
The Periodic Table
... As electricity flows through the ions, electrons and neutral particles, electrons are transferred to higher energy levels and a photon of light is emitted (given off) as the electron returns to its original energy state or ground state. In fog lights, sodium plasma emits a yellow glowing light. Th ...
... As electricity flows through the ions, electrons and neutral particles, electrons are transferred to higher energy levels and a photon of light is emitted (given off) as the electron returns to its original energy state or ground state. In fog lights, sodium plasma emits a yellow glowing light. Th ...
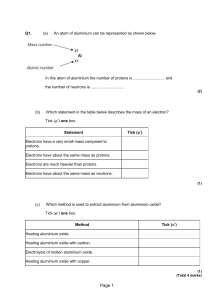
c2 atomic structure f pmh
... Electrons have a very small mass compared to protons. Electrons have about the same mass as protons. Electrons are much heavier than protons. Electrons have about the same mass as neutrons. ...
... Electrons have a very small mass compared to protons. Electrons have about the same mass as protons. Electrons are much heavier than protons. Electrons have about the same mass as neutrons. ...
Page | 1 MATS1101 Chemistry notes semester 2 2012 TOPIC 1
... A mole of a substance is its atomic, molecular or formula mass expressed in grams Amu = atomic mass unit, where 1amu= 1/12 mass of 1 atom of Carbon-12 (Unit also called the Dalton ...
... A mole of a substance is its atomic, molecular or formula mass expressed in grams Amu = atomic mass unit, where 1amu= 1/12 mass of 1 atom of Carbon-12 (Unit also called the Dalton ...
Matter
... idea that matter is formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means “uncuttable,” for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The Greek idea of atoms had to wait about 2,000 years before it ...
... idea that matter is formed of small pieces that could not be cut into smaller parts. He used the word atomos, which means “uncuttable,” for these smallest possible pieces. In modern terms, an atom is the smallest particle of an element. The Greek idea of atoms had to wait about 2,000 years before it ...
Chemical History for L3
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of other elements. 3. Atoms of different elements can chemically combine with one another in small whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, join ...
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of other elements. 3. Atoms of different elements can chemically combine with one another in small whole-number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, join ...
Stoichiometry and Balanced Reactions Chemical Accounting
... – Fortunately, stoichiometric ratios apply for larger numbers of molecules (dozens, hundreds, millions…and more – In the laboratory, it is more practical to do things in terms of mass or volume, which are easy to measure. • How might we translate from one to the other? • Mass of an atom (atomic mass ...
... – Fortunately, stoichiometric ratios apply for larger numbers of molecules (dozens, hundreds, millions…and more – In the laboratory, it is more practical to do things in terms of mass or volume, which are easy to measure. • How might we translate from one to the other? • Mass of an atom (atomic mass ...
Atomic Structure
... • The ___________of the atom • Contains ______________________of the atom • ____________ and ____________ are found ______ the nucleus of an atom Mass Number =___________________________ ...
... • The ___________of the atom • Contains ______________________of the atom • ____________ and ____________ are found ______ the nucleus of an atom Mass Number =___________________________ ...
Dec. 15 , 2012, 9:00 am – noon - Dr. K. Brown
... E) No way of knowing with information given 17) A 1.00 L flask is filled with 0.160 g of unknown gas at 743 mmHg and 25 0C. Calculate the molar mass and identify the gas. The unknown gas is: A) CO2 B) O2 C) Ne D) He E) can be any of the above 18) Oxygen gas, generated by the reaction 2 KClO3 (s) → 2 ...
... E) No way of knowing with information given 17) A 1.00 L flask is filled with 0.160 g of unknown gas at 743 mmHg and 25 0C. Calculate the molar mass and identify the gas. The unknown gas is: A) CO2 B) O2 C) Ne D) He E) can be any of the above 18) Oxygen gas, generated by the reaction 2 KClO3 (s) → 2 ...
Document
... Relative atomic mass It is a dimensionless physical quantity, the ratio of the average mass of atoms of an element (from a single given sample or source) to 1/12 of the mass of an atom of carbon-12. The amount of substance: The SI unit for amount of substance is the mole. The mole is defined as the ...
... Relative atomic mass It is a dimensionless physical quantity, the ratio of the average mass of atoms of an element (from a single given sample or source) to 1/12 of the mass of an atom of carbon-12. The amount of substance: The SI unit for amount of substance is the mole. The mole is defined as the ...