b. matching
... Atomic radii generally _____________________________________ as you move from left to right in a period. Atomic size generally _______________________________ within a given group because there are more ___________________________ occupied and an increased shielding effect, despite an increase in nu ...
... Atomic radii generally _____________________________________ as you move from left to right in a period. Atomic size generally _______________________________ within a given group because there are more ___________________________ occupied and an increased shielding effect, despite an increase in nu ...
File
... except for Period 1. Across a period from left to right, the elements become ___________________ and ___________________ in their properties. o Most ____________________________________ are on the left side of the table (group 1) o Most ____________________________________ are on the right side (gro ...
... except for Period 1. Across a period from left to right, the elements become ___________________ and ___________________ in their properties. o Most ____________________________________ are on the left side of the table (group 1) o Most ____________________________________ are on the right side (gro ...
hc1(5)notes
... • In the periodic table, the f-block elements are wedged between Groups 2 and 3 in the sixth and seventh periods. • Their position reflects the fact that they involve the filling of the 4f sublevel. • The first row of the f block, the lanthanides, are shiny metals similar in reactivity to the Group ...
... • In the periodic table, the f-block elements are wedged between Groups 2 and 3 in the sixth and seventh periods. • Their position reflects the fact that they involve the filling of the 4f sublevel. • The first row of the f block, the lanthanides, are shiny metals similar in reactivity to the Group ...
The Periodic Table
... Mendeleev was the first scientist to notice the relationship between the elements ...
... Mendeleev was the first scientist to notice the relationship between the elements ...
Chapter 6 Powerpoint
... 1. Into what four classes can elements be sorted based on their electron configuration? 2. Why do the elements potassium and sodium have similar chemical properties? ...
... 1. Into what four classes can elements be sorted based on their electron configuration? 2. Why do the elements potassium and sodium have similar chemical properties? ...
Chapter 5 Chem classnotes
... from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. IE increases left to right (across a period) and decrease top to bot ...
... from a neutral atom of an element. As atomic number increases going down a group, more electrons lie between the nucleus and the electrons in the outer orbits. This shields the outer electrons from the nuclear forces of attraction. IE increases left to right (across a period) and decrease top to bot ...
Ex. 06 Answer
... c) i) Their atoms have different number of occupied electron shells. ii) Their atoms have the same number of outermost shell electrons. d) Both of them react with water vigorously. Hydrogen and an alkaline solution are formed in both cases. e) The balloon will fall because the density of krypton is ...
... c) i) Their atoms have different number of occupied electron shells. ii) Their atoms have the same number of outermost shell electrons. d) Both of them react with water vigorously. Hydrogen and an alkaline solution are formed in both cases. e) The balloon will fall because the density of krypton is ...
The Periodic Table
... Transition elements have properties similar to one another and to other metals, but their properties do not fit in with those of any other group. Many transition metals combine chemically with oxygen to form compounds called oxides. ...
... Transition elements have properties similar to one another and to other metals, but their properties do not fit in with those of any other group. Many transition metals combine chemically with oxygen to form compounds called oxides. ...
The Periodic Table
... Transition elements have properties similar to one another and to other metals, but their properties do not fit in with those of any other group. Many transition metals combine chemically with oxygen to form compounds called oxides. ...
... Transition elements have properties similar to one another and to other metals, but their properties do not fit in with those of any other group. Many transition metals combine chemically with oxygen to form compounds called oxides. ...
MENDELEEV`S PERIODIC TABLE
... table are arranged according to atomic number (the number of protons in an element). This fixed Mendeleev’s problem with some elements not fitting the pattern when arranged by atomic mass. Summary Mendeleev transformed an earlier understanding about the classification of elements into a grand plan ...
... table are arranged according to atomic number (the number of protons in an element). This fixed Mendeleev’s problem with some elements not fitting the pattern when arranged by atomic mass. Summary Mendeleev transformed an earlier understanding about the classification of elements into a grand plan ...
mendeleev*s periodic table
... table are arranged according to atomic number (the number of protons in an element). This fixed Mendeleev’s problem with some elements not fitting the pattern when arranged by atomic mass. Summary Mendeleev transformed an earlier understanding about the classification of elements into a grand plan ...
... table are arranged according to atomic number (the number of protons in an element). This fixed Mendeleev’s problem with some elements not fitting the pattern when arranged by atomic mass. Summary Mendeleev transformed an earlier understanding about the classification of elements into a grand plan ...
Study Guide
... a column in the periodic table; elements in the same family will have similar properties (same as family) ...
... a column in the periodic table; elements in the same family will have similar properties (same as family) ...
Give the name and symbol for the element found in
... Dmitri Mendeleev was a Russian scientist credited with creating the first periodic table. He arranged all of the known elements in order of increasing atomic mass. He placed the elements in rows and columns so that elements with similar properties would be located together. He left blank spaces in h ...
... Dmitri Mendeleev was a Russian scientist credited with creating the first periodic table. He arranged all of the known elements in order of increasing atomic mass. He placed the elements in rows and columns so that elements with similar properties would be located together. He left blank spaces in h ...
Ch. 6 SG answers
... 10. Explain the distinction between atomic mass and atomic number. Mass – is the mass of protons and neutron mass atomic number is just # of protons 11. In the periodic table, the atomic masses of Te and I decrease rather than increase, while their atomic numbers increase. This phenomenon happens to ...
... 10. Explain the distinction between atomic mass and atomic number. Mass – is the mass of protons and neutron mass atomic number is just # of protons 11. In the periodic table, the atomic masses of Te and I decrease rather than increase, while their atomic numbers increase. This phenomenon happens to ...
18HYD13_F_Layout 1
... Classification means grouping of elements on the basis of similarities and properties. It is difficult to study each and every element individually and to know their properties and uses. Therefore, they have been classified into groups on the basis of their similarities. Dobereiner’ Triads: When the ...
... Classification means grouping of elements on the basis of similarities and properties. It is difficult to study each and every element individually and to know their properties and uses. Therefore, they have been classified into groups on the basis of their similarities. Dobereiner’ Triads: When the ...
vocab - SALAZAR!!
... Sentence: The Lanthanide series is the second row from the bottom. 6. Actinide series- The actinide series is much different. They are all radioactive and some are not found in nature. Sentence: The Actinide series is very reactive. 7. Noble Gas- The noble gases make a group of chemical elements wit ...
... Sentence: The Lanthanide series is the second row from the bottom. 6. Actinide series- The actinide series is much different. They are all radioactive and some are not found in nature. Sentence: The Actinide series is very reactive. 7. Noble Gas- The noble gases make a group of chemical elements wit ...
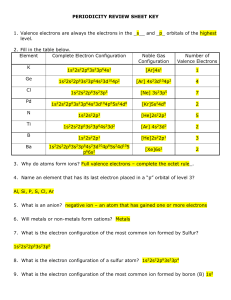
periodicity review sheet key
... 11. What noble gas has the same configuration as a Mg2+ ion? Neon 12. What noble gas has the same configuration as an O2- ion? Neon 13. What is the Shielding Effect? Attraction between the nucleus of an atom and the valence electrons is shielded by levels of electrons in between ...
... 11. What noble gas has the same configuration as a Mg2+ ion? Neon 12. What noble gas has the same configuration as an O2- ion? Neon 13. What is the Shielding Effect? Attraction between the nucleus of an atom and the valence electrons is shielded by levels of electrons in between ...
Name
... 7. Its most common oxidation state is -2 _________________ 8. A metal with more than one oxidation state ________________ 9. Metal with an oxidation number of +3 _________________ 10. Has oxidation numbers of + 1 and -1 _________________ ...
... 7. Its most common oxidation state is -2 _________________ 8. A metal with more than one oxidation state ________________ 9. Metal with an oxidation number of +3 _________________ 10. Has oxidation numbers of + 1 and -1 _________________ ...
The History and Arrangement of the Periodic Table
... density, color, melting point and valence number). ...
... density, color, melting point and valence number). ...
20161025131513
... o # of known elements at his timeo what inspired his approach to the periodic tableo made a “deck of cards” o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodi ...
... o # of known elements at his timeo what inspired his approach to the periodic tableo made a “deck of cards” o what patterns did he noticeo what was his final arrangement of the periodic tableo what was missing in his tableo how did he predict undiscovered elementso was he the first to make a periodi ...
REVIEW Through Course Task
... 10. While metals come in many colors, most at the far left side of the periodic table are very ____________ in color and tend to become more _________ or darker colored toward the center SILVER DULL and right side of the table. 11. While, as a general rule, most metals have high melting points, ther ...
... 10. While metals come in many colors, most at the far left side of the periodic table are very ____________ in color and tend to become more _________ or darker colored toward the center SILVER DULL and right side of the table. 11. While, as a general rule, most metals have high melting points, ther ...
Bohr Model Activity
... e. Use a different colour to highlight the electrons in the outermost shell. ...
... e. Use a different colour to highlight the electrons in the outermost shell. ...
Group 3 element

Group 3 is a group of elements in the periodic table. This group, like other d-block groups, should contain four elements, but it is not agreed what elements belong in the group. Scandium (Sc) and yttrium (Y) are always included, but the other two spaces are usually occupied by lanthanum (La) and actinium (Ac), or by lutetium (Lu) and lawrencium (Lr); less frequently, it is considered the group should be expanded to 32 elements (with all the lanthanides and actinides included) or contracted to contain only scandium and yttrium. The group itself has not acquired a trivial name; however, scandium, yttrium and the lanthanides are sometimes called rare earth metals.Three group 3 elements occur naturally, scandium, yttrium, and either lanthanum or lutetium. Lanthanum continues the trend started by two lighter members in general chemical behavior, while lutetium behaves more similarly to yttrium. This is in accordance with the trend for period 6 transition metals to behave more similarly to their upper periodic table neighbors. This trend is seen from hafnium, which is almost identical chemically to zirconium, to mercury, which is quite distant chemically from cadmium, but still shares with it almost equal atomic size and other similar properties. They all are silvery-white metals under standard conditions. The fourth element, either actinium or lawrencium, has only radioactive isotopes. Actinium, which occurs only in trace amounts, continues the trend in chemical behavior for metals that form tripositive ions with a noble gas configuration; synthetic lawrencium is calculated and partially shown to be more similar to lutetium and yttrium. So far, no experiments have been conducted to synthesize any element that could be the next group 3 element. Unbiunium (Ubu), which could be considered a group 3 element if preceded by lanthanum and actinium, might be synthesized in the near future, it being only three spaces away from the current heaviest element known, ununoctium.