Periodic Table
... The term noble gas comes from the fact that, just like the common view of human nobility, these gases generally sit around not doing anything, and avoid reacting with 'common' elements. The noble gases were previously referred to as inert gases, but this term is not strictly accurate now that some h ...
... The term noble gas comes from the fact that, just like the common view of human nobility, these gases generally sit around not doing anything, and avoid reacting with 'common' elements. The noble gases were previously referred to as inert gases, but this term is not strictly accurate now that some h ...
Ch.4 Notes Powerpoint Version
... • Group 7(A) (or 17) = Halogens. • Group 8(A) (or 18) = Noble Gases. • Remember: the group/column number tells you how many valence electrons those elements have! ...
... • Group 7(A) (or 17) = Halogens. • Group 8(A) (or 18) = Noble Gases. • Remember: the group/column number tells you how many valence electrons those elements have! ...
The Periodic Table - Brookwood High School
... Most group A and all group B elements are metals Group 1A elements (except H) are alkali metals Group 2A elements are alkaline earth metals; both are chemically reactive, alkali the more reactive Elements from group B (lanthanide series) are used as phosphors, substances that emit light when struck ...
... Most group A and all group B elements are metals Group 1A elements (except H) are alkali metals Group 2A elements are alkaline earth metals; both are chemically reactive, alkali the more reactive Elements from group B (lanthanide series) are used as phosphors, substances that emit light when struck ...
The Periodic Table
... are not alike in properties. have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely a ...
... are not alike in properties. have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely a ...
The Periodic Table
... are not alike in properties. have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely a ...
... are not alike in properties. have similar but not In fact, the properties identical properties. change greatly across even For example, lithium (Li), given row. sodium (Na), potassium (K), and other members of The first element in a period is always an group IA are all soft, white, extremely a ...
10C The Periodic Table
... elements. The periodic table shows five basic pieces of information. Four are labeled on the graphic at right; the fifth piece of information is the location of the element in the table itself. The location shows the element group, chemical behavior, approximate atomic mass and size, and other chara ...
... elements. The periodic table shows five basic pieces of information. Four are labeled on the graphic at right; the fifth piece of information is the location of the element in the table itself. The location shows the element group, chemical behavior, approximate atomic mass and size, and other chara ...
Periodic table of elements
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
... One important property of the noble gases is their inactivity. They are inactive because their outermost energy level is full. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, ...
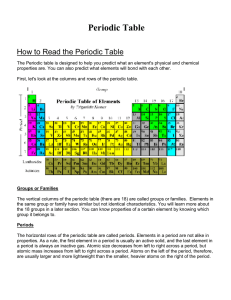
How to Read the Periodic Table
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
(periods) to
... • The table is a visual representation of the periodic law which states that certain properties of elements repeat periodically when arranged by atomic number. • In the standard periodic table, the elements are listed in order of increasing atomic number (the number of protons in the nucleus of an ...
... • The table is a visual representation of the periodic law which states that certain properties of elements repeat periodically when arranged by atomic number. • In the standard periodic table, the elements are listed in order of increasing atomic number (the number of protons in the nucleus of an ...
Document
... Which of the following chlorides is likely to have the most ionic character? LiCl CsCl BeCl2 CaCl2 ...
... Which of the following chlorides is likely to have the most ionic character? LiCl CsCl BeCl2 CaCl2 ...
Topic 5: The periodic Table
... • Elements are everywhere – even in your mouth! • Stainless steel braces are made of carbon, nickel, and iron. • Elements used in braces must be strong enough to straighten teeth but unreactive enough that a chemical reaction won’t occur with mild acids and gases that are inhaled and exhaled. • The ...
... • Elements are everywhere – even in your mouth! • Stainless steel braces are made of carbon, nickel, and iron. • Elements used in braces must be strong enough to straighten teeth but unreactive enough that a chemical reaction won’t occur with mild acids and gases that are inhaled and exhaled. • The ...
File
... 1. Explain why it is more useful to display the elements as a periodic table than as a list. 2. The periodic table is an arrangement of all the known elements. What information is given by the group and period numbers on the periodic table? 3. Explain why water does not appear in the periodic table. ...
... 1. Explain why it is more useful to display the elements as a periodic table than as a list. 2. The periodic table is an arrangement of all the known elements. What information is given by the group and period numbers on the periodic table? 3. Explain why water does not appear in the periodic table. ...
Develo ment of the Atomic Theo John Dalton - (English 1766
... elements appeared to be misplaced in terms of their properties. He assumed that their atomic mass was just calculated incorrectly. 50 yrs. later a British scientist - Henry Moseley discovered the atomic numbers of the elements. He noticed that when the elements were rearranged in order of increasing ...
... elements appeared to be misplaced in terms of their properties. He assumed that their atomic mass was just calculated incorrectly. 50 yrs. later a British scientist - Henry Moseley discovered the atomic numbers of the elements. He noticed that when the elements were rearranged in order of increasing ...
Physical Science 100 Chapter 18, The Periodic Table
... Mendeleev created a table with elements arranged by atomic weights. He noted behaviors of chemical properties that appeared to be periodic in weights. Chemical Similarity in Families • chemical behavior is dictated by the “valence” electrons • the number of valence electrons is the same for elements ...
... Mendeleev created a table with elements arranged by atomic weights. He noted behaviors of chemical properties that appeared to be periodic in weights. Chemical Similarity in Families • chemical behavior is dictated by the “valence” electrons • the number of valence electrons is the same for elements ...
CBSE Class 10 Physics Periodic classification of elements Notes
... b. It contained only 56 elements. Further it was assumed by Newlands that only 56 elements existed in nature and no more elements would be discovered in the future. c. In order to fit elements into the table. Newlands’ adjusted two elements in the same slot and also put some unlike elements under sa ...
... b. It contained only 56 elements. Further it was assumed by Newlands that only 56 elements existed in nature and no more elements would be discovered in the future. c. In order to fit elements into the table. Newlands’ adjusted two elements in the same slot and also put some unlike elements under sa ...
word - My eCoach
... energy. Plot the ionization energy (y-axis) of the first 54 elements against their atomic number (x-axis). 2. Label each point on the graph with the symbol of the element. Analysis and Conclusion 1. Describe the general shape of the graph. ...
... energy. Plot the ionization energy (y-axis) of the first 54 elements against their atomic number (x-axis). 2. Label each point on the graph with the symbol of the element. Analysis and Conclusion 1. Describe the general shape of the graph. ...
Chem 115 POGIL Worksheet
... may not all be the same. Indeed, with a few exceptions, most naturally occurring samples of an element are mixtures of two or more isotopes in unequal portions. We generally deal with samples containing large numbers of atoms with the usual mix of isotopes for the element, so it is more useful to us ...
... may not all be the same. Indeed, with a few exceptions, most naturally occurring samples of an element are mixtures of two or more isotopes in unequal portions. We generally deal with samples containing large numbers of atoms with the usual mix of isotopes for the element, so it is more useful to us ...
Chemistry 4.1 Atomic structure and the periodic table NEED TO
... The boiling points of the noble gases increase with increasing relative atomic mass (going down the group). The elements in Group 1 of the periodic table, known as the alkali metals: are metals with low density (the first three elements in the group are less dense than water) react with non-meta ...
... The boiling points of the noble gases increase with increasing relative atomic mass (going down the group). The elements in Group 1 of the periodic table, known as the alkali metals: are metals with low density (the first three elements in the group are less dense than water) react with non-meta ...
FREE Sample Here
... positive charges, and so on. Table 2.3 can be challenging for students. A tip for learning the table is to indicate that the charges on alkali metal ions (Group 1A) can only be +1 and that the charges on alkaline earth metal ions (Group 2A) can only be +2. Another important point to note is that the ...
... positive charges, and so on. Table 2.3 can be challenging for students. A tip for learning the table is to indicate that the charges on alkali metal ions (Group 1A) can only be +1 and that the charges on alkaline earth metal ions (Group 2A) can only be +2. Another important point to note is that the ...
The Periodic Table
... 3. Atomic Number, Mass Number, Atomic Mass, Atomic Mass Unit, isotope, ion (definitions – make flash cards for these!!!) Atom Foldable – study well!! IPPEX: The Atom (skim over for review but don’t spend much time here) Nucleus Numbers – do not study Isotopes – know definition of isotope and ...
... 3. Atomic Number, Mass Number, Atomic Mass, Atomic Mass Unit, isotope, ion (definitions – make flash cards for these!!!) Atom Foldable – study well!! IPPEX: The Atom (skim over for review but don’t spend much time here) Nucleus Numbers – do not study Isotopes – know definition of isotope and ...
Chapter 2 - Test Bank
... diatomic molecules. Examples of diatomic molecules include N2, O2, F2, Cl2, Br2, and I2. The vast majority of molecules contain more than two atoms classified as polyatomic molecules. Ozone, O3, is a well-known example. It should be pointed out that polyatomic molecules are not required to have only ...
... diatomic molecules. Examples of diatomic molecules include N2, O2, F2, Cl2, Br2, and I2. The vast majority of molecules contain more than two atoms classified as polyatomic molecules. Ozone, O3, is a well-known example. It should be pointed out that polyatomic molecules are not required to have only ...
Chem 115 POGIL Worksheet
... I.U.P.A.C. system designations in parentheses). transition elements - members of the B group elements (North American system), corresponding to the groups 3 through 12 in the I.U.P.A.C. system. The first, second, and third transition series span these groups in periods 4, 5, and 6, respectively. [El ...
... I.U.P.A.C. system designations in parentheses). transition elements - members of the B group elements (North American system), corresponding to the groups 3 through 12 in the I.U.P.A.C. system. The first, second, and third transition series span these groups in periods 4, 5, and 6, respectively. [El ...
Chapter 5—The Periodic Law
... Group a. 3. c. 10. b. 7. d. 17. 61. Titanium, atomic number 22, has the configuration [Ar] 3d2 4s2. To what group does titanium belong? a. Group 2 c. Group 4 b. Group 3 d. Group 5 65. Bromine, atomic number 35, belongs to Group 17. How many electrons does bromine have in its outermost energy level? ...
... Group a. 3. c. 10. b. 7. d. 17. 61. Titanium, atomic number 22, has the configuration [Ar] 3d2 4s2. To what group does titanium belong? a. Group 2 c. Group 4 b. Group 3 d. Group 5 65. Bromine, atomic number 35, belongs to Group 17. How many electrons does bromine have in its outermost energy level? ...
Chapter 6 Review “The Periodic Table”
... that are characterized by the filling of p orbitals are classified as _____. As you move from left to right across the second period of the periodic table, ionization energy __. Atomic size generally decreases as you ____. ...
... that are characterized by the filling of p orbitals are classified as _____. As you move from left to right across the second period of the periodic table, ionization energy __. Atomic size generally decreases as you ____. ...