The Periodical Table and chemical properties
... are found in groups 3-12 of the periodic table. These elements make the transition between the representative metals in groups 1 and 2 and the metalloids, representative metals, and nonmetals in groups 13-18. Moreover, it is in this block of elements in the periodic table that the d-orbitals are bei ...
... are found in groups 3-12 of the periodic table. These elements make the transition between the representative metals in groups 1 and 2 and the metalloids, representative metals, and nonmetals in groups 13-18. Moreover, it is in this block of elements in the periodic table that the d-orbitals are bei ...
The Periodic Table
... Atoms of the same element always have the same number of protons. This identifies them as the element that they are. But all atoms of an element don’t have to have the same number of neutrons. For example, all boron atoms have 5 protons. However, four-fifths of them have 6 neutrons and one-fifth of ...
... Atoms of the same element always have the same number of protons. This identifies them as the element that they are. But all atoms of an element don’t have to have the same number of neutrons. For example, all boron atoms have 5 protons. However, four-fifths of them have 6 neutrons and one-fifth of ...
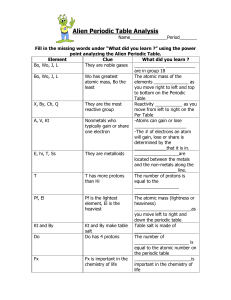
CHMR_AYS_U4AlienPeriodicTableAnalysis_V01
... chemistry of life. The further to the right you move on the Periodic Table, the __________ _____________ the element is. As you move across the periodic table from left to right, the number of electrons ______________ in period. Elements on the right side of the periodic table ___________ electrons ...
... chemistry of life. The further to the right you move on the Periodic Table, the __________ _____________ the element is. As you move across the periodic table from left to right, the number of electrons ______________ in period. Elements on the right side of the periodic table ___________ electrons ...
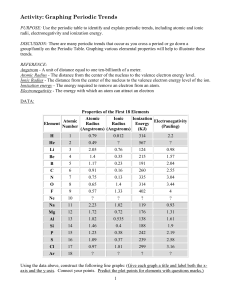
LSHS Graphing Periodic Trends Lab
... The [ p+, N , e- ] in the nucleus increase, thus pulling the [ p+ , N , e- ] closer towards the center of the atom and [ increasing, decreasing ] the atomic radii. Because of this increase in electromagnetic, strong force atoms tend to [ gain, lose] electrons as you go across the periodic table. 9. ...
... The [ p+, N , e- ] in the nucleus increase, thus pulling the [ p+ , N , e- ] closer towards the center of the atom and [ increasing, decreasing ] the atomic radii. Because of this increase in electromagnetic, strong force atoms tend to [ gain, lose] electrons as you go across the periodic table. 9. ...
Periodic Properties of the Elements
... 1. In Exploration 1, select the element from period 2, group 1, on the periodic table. When selected, the group and period number will be highlighted by red circles. 2. Select the element’s period (row) number, represented by a red circle. 3. Record the element’s properties from the list provided in ...
... 1. In Exploration 1, select the element from period 2, group 1, on the periodic table. When selected, the group and period number will be highlighted by red circles. 2. Select the element’s period (row) number, represented by a red circle. 3. Record the element’s properties from the list provided in ...
reviewing key trends
... 5. What is the difference between a substance’s first ionization energy and the second ionization energy? 6. Use Figure 14-13 to assist with these: a) How do the values for the first, second, and third ionization energies compare for the element ‘oxygen’? b) Are there any elements that don’t follow ...
... 5. What is the difference between a substance’s first ionization energy and the second ionization energy? 6. Use Figure 14-13 to assist with these: a) How do the values for the first, second, and third ionization energies compare for the element ‘oxygen’? b) Are there any elements that don’t follow ...
Study Material - Tiwariacademy.net
... Mendeleev's periodic law :– The properties of elements are the periodic function of their atomic mass. Mendeleev's periodic table based on the chemical properties of elements. Contain eight vertical columns called groups and seven horizontal rows called periods form Mendeleev’s peridic table. Achiev ...
... Mendeleev's periodic law :– The properties of elements are the periodic function of their atomic mass. Mendeleev's periodic table based on the chemical properties of elements. Contain eight vertical columns called groups and seven horizontal rows called periods form Mendeleev’s peridic table. Achiev ...
Unit 4 Pack
... Vocab Quiz: Friday, Oct. 3 Test Date: Wednesday, Oct. 8 Vocabulary: average atomic mass metal Mendeleev ionization energy alkali metals actinides ...
... Vocab Quiz: Friday, Oct. 3 Test Date: Wednesday, Oct. 8 Vocabulary: average atomic mass metal Mendeleev ionization energy alkali metals actinides ...
Page 1
... all its outermost electrons. The net positive charge on the ion ensures that all the electrons in the ion are strongly attracted to the nucleus, keeping the ion small. Group VIIIA (18) are highest; Group IA (1) are the lowest. It is the energy released when a neutral atom gains an electron to form a ...
... all its outermost electrons. The net positive charge on the ion ensures that all the electrons in the ion are strongly attracted to the nucleus, keeping the ion small. Group VIIIA (18) are highest; Group IA (1) are the lowest. It is the energy released when a neutral atom gains an electron to form a ...
THE PERIODIC TABLE The Periodic Table lists all known
... There are also trends in successive ionisation energies (i.e. in second, third, fourth, etc). If you ionise an atom one electron at a time, each electron will require more energy to remove than the one before. There are a few reasons for this. Firstly, it is always harder to remove an electron from ...
... There are also trends in successive ionisation energies (i.e. in second, third, fourth, etc). If you ionise an atom one electron at a time, each electron will require more energy to remove than the one before. There are a few reasons for this. Firstly, it is always harder to remove an electron from ...
Atomic size - McKnightScience
... is added to toothpaste Chlorine gas is a deathly green gas that was first used as a form of chemical WARFARE in WWII ...
... is added to toothpaste Chlorine gas is a deathly green gas that was first used as a form of chemical WARFARE in WWII ...
Alien Periodic Table Analysis
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
MENDELEEV`S PERIODIC TABLE
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
mendeleev*s periodic table
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
Chapter 5 The Periodic Law
... a. Without looking at the periodic table, identify the group, period, and block in which the element that has the electron configuration [Xe]6s2 is located. b. Without looking at the periodic table, write the electron configuration for the Group 1 element in the third period. Is this element likely ...
... a. Without looking at the periodic table, identify the group, period, and block in which the element that has the electron configuration [Xe]6s2 is located. b. Without looking at the periodic table, write the electron configuration for the Group 1 element in the third period. Is this element likely ...
Chapter 7 Periodic Properties of Elements - GCG-42
... solids and reactive non-metals. Each period ends with a non-reactive noble gas ...
... solids and reactive non-metals. Each period ends with a non-reactive noble gas ...
AP Chemistry Chapter 7
... • Made some exceptions to place elements in rows with similar properties (Te and I) • Horizontal rows have similar chemical properties • Gaps for “yet to be discovered” elements • Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavio ...
... • Made some exceptions to place elements in rows with similar properties (Te and I) • Horizontal rows have similar chemical properties • Gaps for “yet to be discovered” elements • Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavio ...
hc1(5)notes
... • Atoms tend to be smaller the farther to the right they are found across a period. • The trend to smaller atoms across a period is caused by the (increasing / decreasing) positive charge of the nucleus, which attracts electrons toward the nucleus. • Atoms tend to be larger the farther down in a gro ...
... • Atoms tend to be smaller the farther to the right they are found across a period. • The trend to smaller atoms across a period is caused by the (increasing / decreasing) positive charge of the nucleus, which attracts electrons toward the nucleus. • Atoms tend to be larger the farther down in a gro ...
The Periodic Table
... • They have the same number of valence electrons. • They will form the same kinds of ions. • They increase in size from smallest at top to largest at bottom. ...
... • They have the same number of valence electrons. • They will form the same kinds of ions. • They increase in size from smallest at top to largest at bottom. ...
File
... Metalloids are in the middle of the periodic table and only include elements in which a whole side touched the staircase. ...
... Metalloids are in the middle of the periodic table and only include elements in which a whole side touched the staircase. ...
Chapter 2 Elements are made up of small particles called atoms. All
... The periodic table is a chart where elements are organized depending on how similar they are. The horizontal rows are called periods and the vertical columns are called groups or families. ...
... The periodic table is a chart where elements are organized depending on how similar they are. The horizontal rows are called periods and the vertical columns are called groups or families. ...
Atoms - Schoolwires.net
... • Metals-shiny,smooth, solid at room temperature, good conductors of heat and electricity, malleable and ductile. • Metalloids (along stair step line) physical and chemical properties of both metals and nonmetals- B, Si, Ge, As, Sb, Te • Nonmetals-low melting and boiling points, brittle, dull-lookin ...
... • Metals-shiny,smooth, solid at room temperature, good conductors of heat and electricity, malleable and ductile. • Metalloids (along stair step line) physical and chemical properties of both metals and nonmetals- B, Si, Ge, As, Sb, Te • Nonmetals-low melting and boiling points, brittle, dull-lookin ...
Periodicity Periodic Table Periodic Properties
... Atomic size generally decreases across a Period. The first ionization energy and electronegativity generally increase across a Period.. This is a result of increasing effective nuclear charge and electrons being in the same principal energy level. ...
... Atomic size generally decreases across a Period. The first ionization energy and electronegativity generally increase across a Period.. This is a result of increasing effective nuclear charge and electrons being in the same principal energy level. ...