Name - Haverford Alchemy
... Active Chemistry –Chapter #1-Fun with the Periodic Table - Activity #7 Goal #2 Relate the positions of elements on the periodic table, their electron arrangements, and their distances from the nearest noble gas, to chemical properties of the elements. 2. a) The noble gases are chemically inactive m ...
... Active Chemistry –Chapter #1-Fun with the Periodic Table - Activity #7 Goal #2 Relate the positions of elements on the periodic table, their electron arrangements, and their distances from the nearest noble gas, to chemical properties of the elements. 2. a) The noble gases are chemically inactive m ...
Periodic Table Presentation Lesson
... Element Properties No two elements will have the same properties. ¨ For example, Calcium has a density of 1.54g/mL with no other element having this same density. ...
... Element Properties No two elements will have the same properties. ¨ For example, Calcium has a density of 1.54g/mL with no other element having this same density. ...
Chapter 11
... and block in which the element that has the electron configuration [Kr]5s1 is located. • 2. a. Without looking at the periodic table, write the group configuration for Group 2 elements • b. Without looking a the periodic table, write the complete electron configuration for the Group 2 elements in th ...
... and block in which the element that has the electron configuration [Kr]5s1 is located. • 2. a. Without looking at the periodic table, write the group configuration for Group 2 elements • b. Without looking a the periodic table, write the complete electron configuration for the Group 2 elements in th ...
Chapter 02
... They are all metals, Ag, Au and Pt are the less reactive Structural materials, paints, catalytic converters, batteries They play important biological roles, e.g., Fe. ...
... They are all metals, Ag, Au and Pt are the less reactive Structural materials, paints, catalytic converters, batteries They play important biological roles, e.g., Fe. ...
"Part 1" Resource
... table, and his first work was published in 1924. This was known as the "Periodic Chart of the Atoms". Into the 1930s the heaviest elements were being put up in the body of the periodic table, and Glenn Seaborg "plucked those out" while working with Fermi in Chicago, naming them the Actinide series, ...
... table, and his first work was published in 1924. This was known as the "Periodic Chart of the Atoms". Into the 1930s the heaviest elements were being put up in the body of the periodic table, and Glenn Seaborg "plucked those out" while working with Fermi in Chicago, naming them the Actinide series, ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
BOOKLETColoring-the-Periodic-Table-Families
... - Metals are good conductor of heat and electricity. - Metals are shinny and ductile (stretchable) - Metals are malleable (can be pounded into thin ...
... - Metals are good conductor of heat and electricity. - Metals are shinny and ductile (stretchable) - Metals are malleable (can be pounded into thin ...
Example
... You can’t play “God” • Law of Conservation of Mass says that we can’t create or destroy mass! • Example: the weight of a piece of paper will be the same as the weight of the ash, ...
... You can’t play “God” • Law of Conservation of Mass says that we can’t create or destroy mass! • Example: the weight of a piece of paper will be the same as the weight of the ash, ...
Periodicity
... elements that were not known yet. He predicted their existence and the properties they should have. These elements were later discovered and the properties he predicted were very accurate for the time. His work “can be thought of as similar to putting together a large puzzle.” (Heath Chemistry) ...
... elements that were not known yet. He predicted their existence and the properties they should have. These elements were later discovered and the properties he predicted were very accurate for the time. His work “can be thought of as similar to putting together a large puzzle.” (Heath Chemistry) ...
Station 1: The Periodic Table 1a. Students know how to relate the
... o One other trend is atomic mass. o As you go from left to right and top to bottom of the periodic table, the atomic mass generally increases. There are some exceptions to this rule. Co to Ni How are elements classified on the periodic table? o There are three classes of elements are 1) metals ...
... o One other trend is atomic mass. o As you go from left to right and top to bottom of the periodic table, the atomic mass generally increases. There are some exceptions to this rule. Co to Ni How are elements classified on the periodic table? o There are three classes of elements are 1) metals ...
periodic trend
... ● Periodicity: regular variations (or patterns) of properties with increasing atomic weight; both chemical and physical properties vary in a periodic (repeating pattern). ● Group: vertical column of elements (“family”) ● Period: horizontal row of elements ...
... ● Periodicity: regular variations (or patterns) of properties with increasing atomic weight; both chemical and physical properties vary in a periodic (repeating pattern). ● Group: vertical column of elements (“family”) ● Period: horizontal row of elements ...
lab19
... easiest electron are periodic functions of their atomic numbers. 2. As the atomic number increases, electrons increase, which causes there to be more valence electrons, and results in a larger atom (larger atomic radius value). 3. As atomic number increases, number of valence electrons increases, th ...
... easiest electron are periodic functions of their atomic numbers. 2. As the atomic number increases, electrons increase, which causes there to be more valence electrons, and results in a larger atom (larger atomic radius value). 3. As atomic number increases, number of valence electrons increases, th ...
Notes: Unit 4: Periodic Table - Mr. Palermo`s Flipped Chemistry
... physical and chemical properties of that element. The elements on the Periodic Table are arranged in order of increasing atomic number. (3.1y) Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. ( ...
... physical and chemical properties of that element. The elements on the Periodic Table are arranged in order of increasing atomic number. (3.1y) Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. ( ...
Unit 2 Exam Review: Matter and its Properties This review does not
... CHEM 5B use the Periodic Table to identify and explain the properties of chemical families, including alkali metals, alkaline earth metals, halogens, noble gases, and transition metals 21. Which elements are designated as noble gases? What is the most significant property of these elements? 22. Thi ...
... CHEM 5B use the Periodic Table to identify and explain the properties of chemical families, including alkali metals, alkaline earth metals, halogens, noble gases, and transition metals 21. Which elements are designated as noble gases? What is the most significant property of these elements? 22. Thi ...
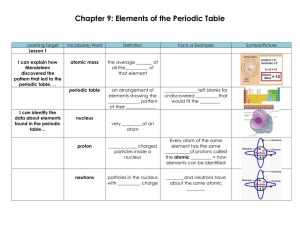
Chapter 9: Elements of the Periodic Table
... I can explain how Mendeleev discovered the pattern that led to the periodic table. . . Mendeleev noticed a ____________ of properties in elements arranged by increasing atomic ___________________. I can identify the data about elements found in the periodic table… The periodic table includes each el ...
... I can explain how Mendeleev discovered the pattern that led to the periodic table. . . Mendeleev noticed a ____________ of properties in elements arranged by increasing atomic ___________________. I can identify the data about elements found in the periodic table… The periodic table includes each el ...
6 The Periodic Tableааааааааааааааааааааааааа__ /__ pts First
... ________ 10. In his periodic table, Mendeleev arranged the elements in order of atomic number. ________ 11. There are six periods in a periodic table. ________ 12. Most of the elements in the periodic table are metals. ________ 13. The elements within a period have similar properties. ...
... ________ 10. In his periodic table, Mendeleev arranged the elements in order of atomic number. ________ 11. There are six periods in a periodic table. ________ 12. Most of the elements in the periodic table are metals. ________ 13. The elements within a period have similar properties. ...
Chapter 2 ATOMS AND ELEMENTS
... • This tells us the mass of one atom of an element relative to one atom of another element. • OR — the mass of 1000 atoms of one relative to 1000 atoms of another. • For example, an O atom is approximately 16 times heavier than an H atom. • Define one element as the standard against which all others ...
... • This tells us the mass of one atom of an element relative to one atom of another element. • OR — the mass of 1000 atoms of one relative to 1000 atoms of another. • For example, an O atom is approximately 16 times heavier than an H atom. • Define one element as the standard against which all others ...
The Periodic Table of Elements
... called Alkali metals - the metals react with water to form alkaline solutions: the solutions turn red litmus paper blue have one outer shell electrons shiny, silvery solids soft, easily cut with scalpel low densities and melting points - these increases down the group good conductors of heat and ele ...
... called Alkali metals - the metals react with water to form alkaline solutions: the solutions turn red litmus paper blue have one outer shell electrons shiny, silvery solids soft, easily cut with scalpel low densities and melting points - these increases down the group good conductors of heat and ele ...
Ch. 6 PPT
... The Modern Periodic Table (cont.) • Vertical columns of elements are called ___________ • Rows of elements are called _________ • Elements in groups 1,2, and 13-18 possess a wide variety of chemical and physical properties and are called the representative elements (or the main group elements). ...
... The Modern Periodic Table (cont.) • Vertical columns of elements are called ___________ • Rows of elements are called _________ • Elements in groups 1,2, and 13-18 possess a wide variety of chemical and physical properties and are called the representative elements (or the main group elements). ...
Regions of the Periodic Table
... noble gases: elements in group VIIIA of the periodic table. 8 valence electrons (except for He which has 2)—full valence shells do not form ions do not react with other compounds ...
... noble gases: elements in group VIIIA of the periodic table. 8 valence electrons (except for He which has 2)—full valence shells do not form ions do not react with other compounds ...
Periodic Table Development
... electrons start to be located in f orbitals f orbitals can hold 14 electrons start filling 4f orbitals on 6 period with La (atomic # 57) f – block elements called inner transition metals Periodic Table shape is due to the way electrons fill s,p,d, f orbitals of different energy levels ...
... electrons start to be located in f orbitals f orbitals can hold 14 electrons start filling 4f orbitals on 6 period with La (atomic # 57) f – block elements called inner transition metals Periodic Table shape is due to the way electrons fill s,p,d, f orbitals of different energy levels ...
Unit 4 - The Periodic Table
... Reactivity: the tendency of an element to react with other elements. Reactivity increases going down for metals, but going up for nonmetals. ...
... Reactivity: the tendency of an element to react with other elements. Reactivity increases going down for metals, but going up for nonmetals. ...
Biology - Mr. Julien`s Homepage
... to B to start a new octet with the last two rows of the representative elements and the p-block elements. c. The International Union of Pure and Applied Chemists numbers the groups from 1 to 18. 2. Metals — ...
... to B to start a new octet with the last two rows of the representative elements and the p-block elements. c. The International Union of Pure and Applied Chemists numbers the groups from 1 to 18. 2. Metals — ...
Week 9 (wk9) - Riverside Local Schools
... 2. In 1860, at the First International Congress of Chemists in Karlsruhe, Germany, Italian chemist Stanislao Cannizzaro presented a convincing method for accurately… 3. Cannizzaro's method enabled chemists to search for relationships between atomic mass and other properties of the… MENDELEEV AND CHE ...
... 2. In 1860, at the First International Congress of Chemists in Karlsruhe, Germany, Italian chemist Stanislao Cannizzaro presented a convincing method for accurately… 3. Cannizzaro's method enabled chemists to search for relationships between atomic mass and other properties of the… MENDELEEV AND CHE ...
The History of the Modern Periodic Table
... Periodic Table • Periodic Table: arrangement of elements in order of their atomic numbers so that elements with similar properties fall in the same column. ...
... Periodic Table • Periodic Table: arrangement of elements in order of their atomic numbers so that elements with similar properties fall in the same column. ...