MATTER AND PERIODIC TABLE
... units (amu). The atomic mass of a single atom is approximately equal to its number of protons plus its number of neutrons. IPC 7D ...
... units (amu). The atomic mass of a single atom is approximately equal to its number of protons plus its number of neutrons. IPC 7D ...
Chapter6
... Mendeleev was able to predict the existence and properties of atomic masses 68 and 70 before they were discovered because he knew that F, Cl, and I must be grouped together. These elements, gallium and germanium, were isolated and discovered in 1875 and 1886 respectively. Mendeleev's predicted prope ...
... Mendeleev was able to predict the existence and properties of atomic masses 68 and 70 before they were discovered because he knew that F, Cl, and I must be grouped together. These elements, gallium and germanium, were isolated and discovered in 1875 and 1886 respectively. Mendeleev's predicted prope ...
PT Trends WS
... Na, Ga, N, and F. Draw the atom (protons, neutrons, and electrons) and ion including electron configuration labeled in the energy levels. Provide the atomic symbol for all. During the formation of an ion what happens to the size of the atomic radius and ...
... Na, Ga, N, and F. Draw the atom (protons, neutrons, and electrons) and ion including electron configuration labeled in the energy levels. Provide the atomic symbol for all. During the formation of an ion what happens to the size of the atomic radius and ...
Periodic Table Presentation Lesson
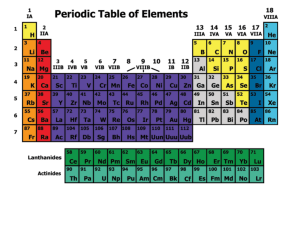
... The elements are arranged on the periodic table according to their atomic numbers. On the table the atomic numbers increase from left to right. ...
... The elements are arranged on the periodic table according to their atomic numbers. On the table the atomic numbers increase from left to right. ...
The Periodic Table
... Atoms of the same element always have the same number of protons. This identifies them as the element that they are. But all atoms of an element don’t have to have the same number of neutrons. For example, all boron atoms have 5 protons. However, four-fifths of them have 6 neutrons and one-fifth of ...
... Atoms of the same element always have the same number of protons. This identifies them as the element that they are. But all atoms of an element don’t have to have the same number of neutrons. For example, all boron atoms have 5 protons. However, four-fifths of them have 6 neutrons and one-fifth of ...
REVIEW TEST 4.5 weeks
... A Material Safety Data Sheet (MSDS) is a document that contains information on the potential health effects of exposure to chemicals, or other potentially dangerous substances, and on safe working procedures when handling chemical products. ...
... A Material Safety Data Sheet (MSDS) is a document that contains information on the potential health effects of exposure to chemicals, or other potentially dangerous substances, and on safe working procedures when handling chemical products. ...
File
... II) The atomic radius decreases with the increasing atomic number across a given period. III) The atomic radius is independent from the type of atom within a given period. IV) Moving from left to right across a given period, there is an increase in the number of protons and electrons. Therefore the ...
... II) The atomic radius decreases with the increasing atomic number across a given period. III) The atomic radius is independent from the type of atom within a given period. IV) Moving from left to right across a given period, there is an increase in the number of protons and electrons. Therefore the ...
Periodic Table notes.notebook
... stable, unreactive elements (although some can form compounds), discovered between 1894‐1900, general valence structure ns2np6, general dot diagram, He is the exception ...
... stable, unreactive elements (although some can form compounds), discovered between 1894‐1900, general valence structure ns2np6, general dot diagram, He is the exception ...
The Periodic Table of the Elements
... • A valence electron for a transition metal is defined as an electron that resides outside a noble-gas core, which can be in an inner or outer shell. • The energies of electrons in the d sublevels are comparable to the s sublevel of the next highest main level (3d and 4s, 4d and 5s), so an element’s ...
... • A valence electron for a transition metal is defined as an electron that resides outside a noble-gas core, which can be in an inner or outer shell. • The energies of electrons in the d sublevels are comparable to the s sublevel of the next highest main level (3d and 4s, 4d and 5s), so an element’s ...
Chapter 6 Test - The Periodic Table
... horizontal row in the periodic table A repetition of properties occurs when elements are arranged in order of increasing atomic number. ...
... horizontal row in the periodic table A repetition of properties occurs when elements are arranged in order of increasing atomic number. ...
Chapter 6 Review “The Periodic Table”
... element in the second period has the largest atomic radius? Which of the following elements is in the same period as phosphorus: a) magnesium, or b) nitrogen? Who arranged the elements according to atomic mass, and used the arrangement to predict the properties of missing elements? ...
... element in the second period has the largest atomic radius? Which of the following elements is in the same period as phosphorus: a) magnesium, or b) nitrogen? Who arranged the elements according to atomic mass, and used the arrangement to predict the properties of missing elements? ...
Periodic Table Development
... To become stable, atoms ______________________ electrons to fill their outermost energy level (valence shell) with 8 electrons (Octet rule). Metals _______ electrons to achieve eight electrons in their valence shell. Nonmetals ________ electrons to achieve eight electrons in their valence shell. Exa ...
... To become stable, atoms ______________________ electrons to fill their outermost energy level (valence shell) with 8 electrons (Octet rule). Metals _______ electrons to achieve eight electrons in their valence shell. Nonmetals ________ electrons to achieve eight electrons in their valence shell. Exa ...
TRENDS in the PERIODIC TABLE
... Going across the 3rd period, the trend for 1st Ionization Energy is to INCREASE. what about Mg to Al then??? ...
... Going across the 3rd period, the trend for 1st Ionization Energy is to INCREASE. what about Mg to Al then??? ...
MENDELEEV`S PERIODIC TABLE
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
mendeleev*s periodic table
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
... grand plan called the periodic table. Mendeleev discovered (with a few exceptions) that arranging elements in order of increasing atomic mass across rows and similar characteric properties down the columns would result in elements obeying the periodic law (they repeated their characteristic proper ...
Power point notes - Social Circle City Schools
... metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and ...
... metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. They are ductile and ...
Electron Energy Level
... Carbon, the graphite in “pencil lead” is a great example of a nonmetallic element. Nonmetals are poor conductors of heat and electricity Nonmetals tend to be brittle Many nonmetals are gases at room temperature ...
... Carbon, the graphite in “pencil lead” is a great example of a nonmetallic element. Nonmetals are poor conductors of heat and electricity Nonmetals tend to be brittle Many nonmetals are gases at room temperature ...
7.1 The Periodic Table
... 7.1 Physical and chemical properties • Characteristics that you can see through direct observation are called physical properties. • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physic ...
... 7.1 Physical and chemical properties • Characteristics that you can see through direct observation are called physical properties. • Physical properties include color, texture, density, brittleness, and state (solid, liquid, or gas). • Melting point, boiling point, and specific heat are also physic ...
Structure of the Atom and Periodic Table Quiz 2016 Self
... 15. Do the vertical columns or the horizontal rows describes elements with similar properties? Support with evidence. The vertical columns, called groups describe elements of similar properties. Groups have names that help describe the properties those elements share. An example if the reactive meta ...
... 15. Do the vertical columns or the horizontal rows describes elements with similar properties? Support with evidence. The vertical columns, called groups describe elements of similar properties. Groups have names that help describe the properties those elements share. An example if the reactive meta ...
Groups
... Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron ...
... Group 8A or 18: Noble Gases cont. Properties 1. all are gases at room temp 2. relatively unreactive Part of the p -block They have no common ionic charge because they don’t form ions, have stable electron ...
Atoms, Elements, and the Periodic Table Spring Packet
... 2. My atomic mass is 35.453. 5. I have 2 electrons in the first shell, 8 in the second shell, and 6 in the third shell. 6. I am the head of the carbon family known as the “basis of life”. 9. My atomic number is 79. 11. I am a transition metal with 25 electrons. 13. I make up 78% of the air and am fo ...
... 2. My atomic mass is 35.453. 5. I have 2 electrons in the first shell, 8 in the second shell, and 6 in the third shell. 6. I am the head of the carbon family known as the “basis of life”. 9. My atomic number is 79. 11. I am a transition metal with 25 electrons. 13. I make up 78% of the air and am fo ...
Lesson 1 - Scientist in Residence
... number is 6. The periodic table is arranged so that the atomic number increases as you read from left to right in a row. The columns are arranged according to common properties the elements exhibit. This is why the table is called periodic: there is a periodicity in the properties of the elements as ...
... number is 6. The periodic table is arranged so that the atomic number increases as you read from left to right in a row. The columns are arranged according to common properties the elements exhibit. This is why the table is called periodic: there is a periodicity in the properties of the elements as ...
Oct 17-Oct 21
... I can use the periodic table to identify the characteristics and properties of metals, nonmetals, and metalloids ...
... I can use the periodic table to identify the characteristics and properties of metals, nonmetals, and metalloids ...
questions on periodic classification of elements
... 11. The elements Li, Be, B , C ,N, O ,F and Ne are present in the second period . How many valence electrons do these elements have? Classify them as metals and non metals. 12. An atom ‘X’ has electronic configuration 2,8,7. a)What is the atomic number of this element? b) State whether ‘X’ is a meta ...
... 11. The elements Li, Be, B , C ,N, O ,F and Ne are present in the second period . How many valence electrons do these elements have? Classify them as metals and non metals. 12. An atom ‘X’ has electronic configuration 2,8,7. a)What is the atomic number of this element? b) State whether ‘X’ is a meta ...
Atoms
... • Metals: usually solid at room temperature, good conductors of heat and electricity, bendable, malleable (can be pounded into sheets), shiny and ductile (can be ...
... • Metals: usually solid at room temperature, good conductors of heat and electricity, bendable, malleable (can be pounded into sheets), shiny and ductile (can be ...