The Periodic Table
... There are 7 periods and 18 groups. Electron config. are repeated in periods. similar e- config. in the same group. Elements in groups are also listed in order of their increasing n. ...
... There are 7 periods and 18 groups. Electron config. are repeated in periods. similar e- config. in the same group. Elements in groups are also listed in order of their increasing n. ...
6.6 – Periodic Table
... Metals – Excellent conductors of heat and electricity. Often ductile (able to be squeezed out into a wire) and malleable (able to be hammered into sheets). Tend to lose electrons (or become positively charged). Can be mixed with other metals to form a mixture called an alloy. Usually found as solids ...
... Metals – Excellent conductors of heat and electricity. Often ductile (able to be squeezed out into a wire) and malleable (able to be hammered into sheets). Tend to lose electrons (or become positively charged). Can be mixed with other metals to form a mixture called an alloy. Usually found as solids ...
PPT Student Notes Blank fill-in
... 1. Took the ____________________charge, which is the number of protons in the nucleus, and ordered the periodic table using the number of protons in the atom. 2. The trends found by Mendeleev were in place. Periodic Law 1. States that physical and chemical properties of elements are properties of th ...
... 1. Took the ____________________charge, which is the number of protons in the nucleus, and ordered the periodic table using the number of protons in the atom. 2. The trends found by Mendeleev were in place. Periodic Law 1. States that physical and chemical properties of elements are properties of th ...
atoms - sciencegeek
... NOTE: Some periodic tables list the mass # on top and atomic # on the bottom while other periodic tables do the reverse. Remember, the smaller of the two numbers is always the atomic number!! ...
... NOTE: Some periodic tables list the mass # on top and atomic # on the bottom while other periodic tables do the reverse. Remember, the smaller of the two numbers is always the atomic number!! ...
Periodictable - Trupia
... Group 2: Alkaline Earth Metals Very active metals, only found in compounds in nature React strongly with water to form hydrogen gas and a base: Ca (s) + 2 H2O (l) Ca(OH)2 (aq) + H2 (g) 2 valence electrons Form +2 ion by losing those valence electrons Form oxides like CaO, MgO, BaO ...
... Group 2: Alkaline Earth Metals Very active metals, only found in compounds in nature React strongly with water to form hydrogen gas and a base: Ca (s) + 2 H2O (l) Ca(OH)2 (aq) + H2 (g) 2 valence electrons Form +2 ion by losing those valence electrons Form oxides like CaO, MgO, BaO ...
16.1 to Lewis dot
... Chemical bonds are formed only between the electrons in the highest unfilled energy level. ...
... Chemical bonds are formed only between the electrons in the highest unfilled energy level. ...
Periodic Table
... Have properties of both metals and nonmetals: - May be shiny or dull - Shape is easily changed - Typically conduct heat and electricity better than nonmetals but not as well as metals - Often used as semiconductors ...
... Have properties of both metals and nonmetals: - May be shiny or dull - Shape is easily changed - Typically conduct heat and electricity better than nonmetals but not as well as metals - Often used as semiconductors ...
Chapter 6 Reading Guide
... 4. What were Dobereiner’s triads? 5. What pattern did he notice about the triads? 6. What are the two reasons Mendeleev gets more credit than Meyer for the periodic table? 7. What approach did Mendelleev take to organizing the elements? 8. How did he eventually organize the elements? 9. What were th ...
... 4. What were Dobereiner’s triads? 5. What pattern did he notice about the triads? 6. What are the two reasons Mendeleev gets more credit than Meyer for the periodic table? 7. What approach did Mendelleev take to organizing the elements? 8. How did he eventually organize the elements? 9. What were th ...
periodic classification - cpprashanths Chemistry
... 5.What are the achievements (merits) of Mendeleev’s periodic table? 1.Systematic study of elements.:- He was the first to arrange elements in to groups and periods which made the study of elements simple. 2.Correction of doubtful atomic masses.:-His periodic table helped in correcting the doubtful ...
... 5.What are the achievements (merits) of Mendeleev’s periodic table? 1.Systematic study of elements.:- He was the first to arrange elements in to groups and periods which made the study of elements simple. 2.Correction of doubtful atomic masses.:-His periodic table helped in correcting the doubtful ...
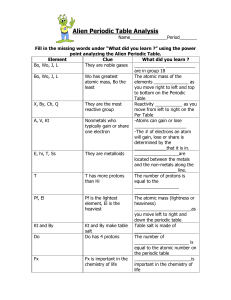
Alien Periodic Table Analysis
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
... They are the most Reactivity ___________ as you reactive group move from left to right on the Per Table A, V, Kt Nonmetals who -Atoms can gain or lose typically gain or share _____________________ one electron -The # of electrons an atom will gain, lose or share is determined by the _____________tha ...
Name - Haverford Alchemy
... Active Chemistry –Chapter #1-Fun with the Periodic Table - Activity #7 Goal #2 Relate the positions of elements on the periodic table, their electron arrangements, and their distances from the nearest noble gas, to chemical properties of the elements. 2. a) The noble gases are chemically inactive m ...
... Active Chemistry –Chapter #1-Fun with the Periodic Table - Activity #7 Goal #2 Relate the positions of elements on the periodic table, their electron arrangements, and their distances from the nearest noble gas, to chemical properties of the elements. 2. a) The noble gases are chemically inactive m ...
Year 10 Chemistry Exam June 2012
... a) may form when two clear solutions are mixed. b) is a soluble salt. c) forms because the ions in a solution repel each other. d) collects at the surface when two solutions are mixed. 14. When solutions of sodium chloride (NaCl) and silver nitrate (AgNO3) are mixed, solid silver chloride (AgCl) for ...
... a) may form when two clear solutions are mixed. b) is a soluble salt. c) forms because the ions in a solution repel each other. d) collects at the surface when two solutions are mixed. 14. When solutions of sodium chloride (NaCl) and silver nitrate (AgNO3) are mixed, solid silver chloride (AgCl) for ...
Unit 9: Periodicity
... electron affinity (electron-liking): the energy change that accompanies the addition of an electron to an atom. – *If energy is released in the process of adding an electron, EA is negative. A negative EA means that the atom wants to gain the electron. The larger the negative number, the more it w ...
... electron affinity (electron-liking): the energy change that accompanies the addition of an electron to an atom. – *If energy is released in the process of adding an electron, EA is negative. A negative EA means that the atom wants to gain the electron. The larger the negative number, the more it w ...
AtomTest
... • To find the number of neutrons in an atom you subtract the atomic number from the atomic mass and round to the nearest whole number. • Atomic Mass – Atomic Number = Neutrons • Use a sheet of paper to solve this problem • Good luck! ...
... • To find the number of neutrons in an atom you subtract the atomic number from the atomic mass and round to the nearest whole number. • Atomic Mass – Atomic Number = Neutrons • Use a sheet of paper to solve this problem • Good luck! ...
Periodic Table Name: Practice Review H
... grouped in A) periods C) horizontal rows E) horizontal families ...
... grouped in A) periods C) horizontal rows E) horizontal families ...
2 periodic table pd9
... Elements with the same Day 6 2-20 # of valence are in the same _______ on the periodic table. alkali metals = __ valence alkaline earth metals = __ valence ...
... Elements with the same Day 6 2-20 # of valence are in the same _______ on the periodic table. alkali metals = __ valence alkaline earth metals = __ valence ...
chem 1411 – chapter 8
... Some elements of the second and third periods which are diagonally positioned, show similarities in their properties. Ex. Li and Mg, Be and Al, B and Si Diagonal relationship is due to the closeness of the charge densities (magnitude of charge / volume) of their cations. Variation in Chemical Proper ...
... Some elements of the second and third periods which are diagonally positioned, show similarities in their properties. Ex. Li and Mg, Be and Al, B and Si Diagonal relationship is due to the closeness of the charge densities (magnitude of charge / volume) of their cations. Variation in Chemical Proper ...
How the Periodic Table Works
... outermost s orbital and the inner d orbital can participate in chemical reactions. They include elements that we usually think of as metals, like iron, nickel, chromium and precious metals such as gold, copper, silver and platinum. Metals are located mostly in group 13 (IIIA) and some in groups 141 ...
... outermost s orbital and the inner d orbital can participate in chemical reactions. They include elements that we usually think of as metals, like iron, nickel, chromium and precious metals such as gold, copper, silver and platinum. Metals are located mostly in group 13 (IIIA) and some in groups 141 ...
Water Metal Hydroxide + Hydrogen
... The atomic radius of an atom is defined as the distance of closest approach to another atom and is the distance at which the mutual repulsion of the electron clouds and the mutual attraction of the nuclear charge of each for the electrons of the other are in equilibrium. The size of an atom in a mol ...
... The atomic radius of an atom is defined as the distance of closest approach to another atom and is the distance at which the mutual repulsion of the electron clouds and the mutual attraction of the nuclear charge of each for the electrons of the other are in equilibrium. The size of an atom in a mol ...
Chapter 11
... • Identify the block, period, group, group name (where appropriate), and element name for the elements with the following electron configurations. • a) [Ne]3s23p1 • b) [Ar]3d104s24p6 • c) [Kr] 4d105s1 • d)Period 4, p group #5 ...
... • Identify the block, period, group, group name (where appropriate), and element name for the elements with the following electron configurations. • a) [Ne]3s23p1 • b) [Ar]3d104s24p6 • c) [Kr] 4d105s1 • d)Period 4, p group #5 ...
Changes in matter
... b)Compare the weight of a specified quantity of matter before and after it undergoes melting or freezing. c)Investigate the results of the combined weights of a liquid and a solid after the solid has been dissolved and then recovered from the liquid (e.g., salt dissolved in water then water evaporat ...
... b)Compare the weight of a specified quantity of matter before and after it undergoes melting or freezing. c)Investigate the results of the combined weights of a liquid and a solid after the solid has been dissolved and then recovered from the liquid (e.g., salt dissolved in water then water evaporat ...
The Periodic Table and Periodic Law
... • The metals of the p block are generally harder and denser than the s-block alkaline-earth metals, but softer and less dense than the dblock metals. • The Noble Gases round out the p block elements and are in general, very un-reactive or inert gases. We have no known compounds of He, Ne, and Ar. ...
... • The metals of the p block are generally harder and denser than the s-block alkaline-earth metals, but softer and less dense than the dblock metals. • The Noble Gases round out the p block elements and are in general, very un-reactive or inert gases. We have no known compounds of He, Ne, and Ar. ...