Pre-class Activity 12/18
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
Chapter6
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
Study Guide
... a column in the periodic table; elements in the same family will have similar properties (same as family) ...
... a column in the periodic table; elements in the same family will have similar properties (same as family) ...
Families of Elements
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
... Elements in group IA of the periodic table, with the exception of hydrogen Have one electron in their outer energy levels Are the most chemically active of all metals (meaning an element readily combines with other substances to form compounds) NEVER found in pure form A way to identify al ...
Chapter 5: What you should know when you finish. Describe the
... Differences in reactivity among the alkaline earth metals are shown by the ways they react with water. Calcium, strontium, and barium react easily with cold water. Magnesium will react with hot water, but no change appears to occur when beryllium is added to water Magnesium and calcium have es ...
... Differences in reactivity among the alkaline earth metals are shown by the ways they react with water. Calcium, strontium, and barium react easily with cold water. Magnesium will react with hot water, but no change appears to occur when beryllium is added to water Magnesium and calcium have es ...
Periodic Table
... Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
... Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
Glossary of Terms Used in Articles about the Hydrogen Economy at
... water, sun or biomass is renewable because the sources are not used up in the process of making energy. Shell In physical chemistry, this term refers to any of the paths or orbits of electrons in an atom as they revolve around its nucleus. There may be from one to seven shells. The number of electro ...
... water, sun or biomass is renewable because the sources are not used up in the process of making energy. Shell In physical chemistry, this term refers to any of the paths or orbits of electrons in an atom as they revolve around its nucleus. There may be from one to seven shells. The number of electro ...
u4ohnotes18f2005 - Teach-n-Learn-Chem
... in-between those of metals and nonmetals “semiconductors” ...
... in-between those of metals and nonmetals “semiconductors” ...
Chemistry: Unit 4 - Teach-n-Learn-Chem
... in-between those of metals and nonmetals “semiconductors” ...
... in-between those of metals and nonmetals “semiconductors” ...
Periodic Table Quiz
... The pictures below show the position of di erent elements on the periodic table. Which picture has an X in the locations of the three elements that would be most similar in the way they react? A. ...
... The pictures below show the position of di erent elements on the periodic table. Which picture has an X in the locations of the three elements that would be most similar in the way they react? A. ...
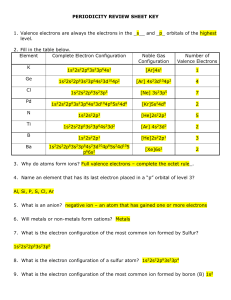
periodicity review sheet key
... c. Electronegativity tends to be higher in the metals than in the nonmetals. d. Electronegativity tends to be lower in the gases than in the solids. 42. The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Which statement accurately describes a pattern in t ...
... c. Electronegativity tends to be higher in the metals than in the nonmetals. d. Electronegativity tends to be lower in the gases than in the solids. 42. The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Which statement accurately describes a pattern in t ...
period 2 - New York Science Teacher
... bottom, the # of electrons in the 2s subshell increases decreases remains the same ...
... bottom, the # of electrons in the 2s subshell increases decreases remains the same ...
9The-Periodic-table1 (3).pptx
... ! More than 75% of elements in the periodic table are metals ! Found to the left of the “staircase” on the periodic table (exception H is not a metal) ! Most metals: ! Shiny/have luster ! ...
... ! More than 75% of elements in the periodic table are metals ! Found to the left of the “staircase” on the periodic table (exception H is not a metal) ! Most metals: ! Shiny/have luster ! ...
Periodic Table Test Review
... 8. What does the atomic number represent? 9. What determines the atomic mass of an element? 10. What are the groups on the Periodic Table and what property determines the group an element is in? 11. What are the periods on the Periodic Table and what determines the period an element is in? 12. What ...
... 8. What does the atomic number represent? 9. What determines the atomic mass of an element? 10. What are the groups on the Periodic Table and what property determines the group an element is in? 11. What are the periods on the Periodic Table and what determines the period an element is in? 12. What ...
Chemistry Midterm Exam Review Sheet
... 35. Why do elements lose or gain electrons? 36. Why do ions have a + or – charge? 37. How many electrons are lost or gained if an ion has the following charge: a. +1 ...
... 35. Why do elements lose or gain electrons? 36. Why do ions have a + or – charge? 37. How many electrons are lost or gained if an ion has the following charge: a. +1 ...
pg156
... 30) Without looking at the periodic table, write the expected outer electron configuration for each of the following elements (Hint: See sample problem 5-3) a. Group 7, fourth period b. Group 3, fifth period c. Group 12, sixth period ...
... 30) Without looking at the periodic table, write the expected outer electron configuration for each of the following elements (Hint: See sample problem 5-3) a. Group 7, fourth period b. Group 3, fifth period c. Group 12, sixth period ...
How are properties of atoms used to organize elements into the
... The Periodic Table • Colour hydrogen and the non-metals yellow • Colour the metals blue • Colour the metalloids green • Make the “staircase” bold • Number the families above them ...
... The Periodic Table • Colour hydrogen and the non-metals yellow • Colour the metals blue • Colour the metalloids green • Make the “staircase” bold • Number the families above them ...
CHMB homework Name © Van Der Sluys, 2004 Periodic Table 1
... 1. Where are the most active metals? _________________________________ 2. Where are the most active nonmetals? ______________________________ 3. As you go from left to right across a period, the atomic size (increases/decreases). 4. As you travel down a group, the atomic size (decreases/increases). ...
... 1. Where are the most active metals? _________________________________ 2. Where are the most active nonmetals? ______________________________ 3. As you go from left to right across a period, the atomic size (increases/decreases). 4. As you travel down a group, the atomic size (decreases/increases). ...
Name: Homeroom
... An element is a substance that contains only one kind of atom. All atoms of an element have the same number of protons also their atomic number. At this time we know more than 110 elements, but only 90 of these occur in nature. Some familiar natural elements are : gold, silver, lead, iron, etc. ...
... An element is a substance that contains only one kind of atom. All atoms of an element have the same number of protons also their atomic number. At this time we know more than 110 elements, but only 90 of these occur in nature. Some familiar natural elements are : gold, silver, lead, iron, etc. ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.