Periodicity PPt
... the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting in Gallipoli by a sniper’s bullet, at the age of 28. Because of this loss, the ...
... the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting in Gallipoli by a sniper’s bullet, at the age of 28. Because of this loss, the ...
the Alkali Metals
... A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same ...
... A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same ...
Chem 115 POGIL Worksheet
... This ordering, however, seemed to place some elements out of sequence. A better arrangement, based on atomic number, became possible in 1913, when Henry G. J. Moseley found that the atomic numbers of elements could be determined experimentally from their characteristic x-ray frequencies. Today the ...
... This ordering, however, seemed to place some elements out of sequence. A better arrangement, based on atomic number, became possible in 1913, when Henry G. J. Moseley found that the atomic numbers of elements could be determined experimentally from their characteristic x-ray frequencies. Today the ...
Atomic Structure and the Periodic Table of Elements: The Secret
... 4. Once you have several distinct piles focusing on one or two shared characteristics, now try looking at the big picture and combine the agents into one large family portrait. 5. If you have compiled the Secret Agents into the correct arrangement, there will be one empty spot in the family portrait ...
... 4. Once you have several distinct piles focusing on one or two shared characteristics, now try looking at the big picture and combine the agents into one large family portrait. 5. If you have compiled the Secret Agents into the correct arrangement, there will be one empty spot in the family portrait ...
Instructional Objectives 3. Atomic Structure and the Periodic Table
... • Describe isotopes of an element how they affect physical and chemical properties. • Describe how an average atomic mass of an element in the periodic table is calculated. Chemistry at a Glance: Atomic Structure and how big is an atom? 3.4 The Periodic Law and the Periodic Table • Describe periodic ...
... • Describe isotopes of an element how they affect physical and chemical properties. • Describe how an average atomic mass of an element in the periodic table is calculated. Chemistry at a Glance: Atomic Structure and how big is an atom? 3.4 The Periodic Law and the Periodic Table • Describe periodic ...
Instructional-Objectives
... Describe isotopes of an element how they affect physical and chemical properties. Describe how an average atomic mass of an element in the periodic table is calculated. Chemistry at a Glance: Atomic Structure and how big is an atom? 3.4 The Periodic Law and the Periodic Table Describe periodic ...
... Describe isotopes of an element how they affect physical and chemical properties. Describe how an average atomic mass of an element in the periodic table is calculated. Chemistry at a Glance: Atomic Structure and how big is an atom? 3.4 The Periodic Law and the Periodic Table Describe periodic ...
Periodic Table Organization Comprehension Questions
... Why does the size of an atom increase as you move from top to bottom on the periodic table? 7. Why does the size of an atom decrease as you move from left to right on the periodic table? 8. Metals, non-metals, and metalloids are grouped on the periodic table. What type of properties do metals share? ...
... Why does the size of an atom increase as you move from top to bottom on the periodic table? 7. Why does the size of an atom decrease as you move from left to right on the periodic table? 8. Metals, non-metals, and metalloids are grouped on the periodic table. What type of properties do metals share? ...
400 Chem periodic table
... • Is the net positive charge experienced by electron in multielectron atom. • Protons always added to nucleus, but electron positions can change as atomic number increases (energy levels). • Three factors control the net charge: size of energy levels, nuclear charge and the shielding effect. • These ...
... • Is the net positive charge experienced by electron in multielectron atom. • Protons always added to nucleus, but electron positions can change as atomic number increases (energy levels). • Three factors control the net charge: size of energy levels, nuclear charge and the shielding effect. • These ...
Chemistry Definitions
... Example: when lighter fluid is burned it changes from a clear liquid to gaseous water and carbon dioxide or when iron corrodes it mixes with other elements (mostly oxygen) When a chemical change occurs, the atoms that make up the substance are rearranged to form a new compound. ...
... Example: when lighter fluid is burned it changes from a clear liquid to gaseous water and carbon dioxide or when iron corrodes it mixes with other elements (mostly oxygen) When a chemical change occurs, the atoms that make up the substance are rearranged to form a new compound. ...
Periodicity Periodic Table Periodic Properties
... General reactivity is highest at the left and right ends of a Period. (Excluding the inert noble gases.) In Period 2, the physical properties change abruptly between carbon (solid) and nitrogen (gas). ...
... General reactivity is highest at the left and right ends of a Period. (Excluding the inert noble gases.) In Period 2, the physical properties change abruptly between carbon (solid) and nitrogen (gas). ...
PT trends
... Developed concept of arranging PT by atomic number Periodic law – there is a periodic repetition of properties when elements are arranged by atomic # ...
... Developed concept of arranging PT by atomic number Periodic law – there is a periodic repetition of properties when elements are arranged by atomic # ...
Unit 1 Review Sheet
... 2. Which element in each pair has the larger first ionization energy? a. Na, K b. Mg, P 3. Explain each of the following comparisons in the terms of nuclear charge and atomic structure. a. Calcium has a smaller second ionization energy than potassium. ...
... 2. Which element in each pair has the larger first ionization energy? a. Na, K b. Mg, P 3. Explain each of the following comparisons in the terms of nuclear charge and atomic structure. a. Calcium has a smaller second ionization energy than potassium. ...
Periodic Table Workshop
... arrangement of elements based on chem. properties of the elements, so that elements with similar properties fell in the same column (62 known in his time) • Brilliant b/c: he left spaces in columns for yet-undiscovered elements ...
... arrangement of elements based on chem. properties of the elements, so that elements with similar properties fell in the same column (62 known in his time) • Brilliant b/c: he left spaces in columns for yet-undiscovered elements ...
How to Read the Periodic Table
... 6. What is the most common type of element on the Periodic Table? (metals, nonmetals, metalloids) 7. What are the physical properties of metals? 8. What are the physical properties of nonmetals? 9. What kind of element would best make a coffee cup that would not burn your hand? 10. What kind of elem ...
... 6. What is the most common type of element on the Periodic Table? (metals, nonmetals, metalloids) 7. What are the physical properties of metals? 8. What are the physical properties of nonmetals? 9. What kind of element would best make a coffee cup that would not burn your hand? 10. What kind of elem ...
Trends on the Periodic Table
... alkaline, or basic, solution, hence their name. Alkaline earth metals (2)—These also are reactive metals, but they don’t explode in water; pastes of these are used in batteries. Halogens (17)—Known as the “salt formers,” they are used in modern lighting and always exist as diatomic molecules in thei ...
... alkaline, or basic, solution, hence their name. Alkaline earth metals (2)—These also are reactive metals, but they don’t explode in water; pastes of these are used in batteries. Halogens (17)—Known as the “salt formers,” they are used in modern lighting and always exist as diatomic molecules in thei ...
The Periodic Law - Mona Shores Blogs
... o Some are so unreactive that they seldom form compounds. Major group are the coinage metals. f-Block Elements: Lanthanides and Actinides Are considered the innertransition metals. f-block is wedged between group 3 and 4 The actinides are all radioactive, and every element past Np is artific ...
... o Some are so unreactive that they seldom form compounds. Major group are the coinage metals. f-Block Elements: Lanthanides and Actinides Are considered the innertransition metals. f-block is wedged between group 3 and 4 The actinides are all radioactive, and every element past Np is artific ...
AP Chemistry Chapter 7
... • Made some exceptions to place elements in rows with similar properties (Te and I) • Horizontal rows have similar chemical properties • Gaps for “yet to be discovered” elements • Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavio ...
... • Made some exceptions to place elements in rows with similar properties (Te and I) • Horizontal rows have similar chemical properties • Gaps for “yet to be discovered” elements • Left questions: why didn’t some elements fit in order of increasing mass? Why did some elements exhibit periodic behavio ...
Periodic Table History & Organization
... atoms that has a charge +/Ionization- process that results in formation of an ion Ionization energy (IE) energy required to remove one electron from a neutral atom Can compare the ease with which atoms give up electrons ...
... atoms that has a charge +/Ionization- process that results in formation of an ion Ionization energy (IE) energy required to remove one electron from a neutral atom Can compare the ease with which atoms give up electrons ...
Ch. 6 SG answers
... b. The physical and chemical properties of the elements are repeating as a result of their atomic number c. Electrons exhibit properties of both particles and waves d. The chemical properties of elements can be group according to their periodicity, but physical properties cannot __B___ 4. Which elem ...
... b. The physical and chemical properties of the elements are repeating as a result of their atomic number c. Electrons exhibit properties of both particles and waves d. The chemical properties of elements can be group according to their periodicity, but physical properties cannot __B___ 4. Which elem ...
Chapter 5 Chem classnotes
... Periodicity or the periodic law states that the physical and chemical properties of the elements are functions of their atomic numbers – when elements are arranged in order of increasing atomic number, elements with similar properties appear at regular intervals. ...
... Periodicity or the periodic law states that the physical and chemical properties of the elements are functions of their atomic numbers – when elements are arranged in order of increasing atomic number, elements with similar properties appear at regular intervals. ...
Periodic Table of Elements
... 2. Among chemical properties, Mendeléev concentrated on the compounds formed by elements with oxygen and hydrogen. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. The formulae of the hydrides and oxides formed by an element were treated as one of th ...
... 2. Among chemical properties, Mendeléev concentrated on the compounds formed by elements with oxygen and hydrogen. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. The formulae of the hydrides and oxides formed by an element were treated as one of th ...
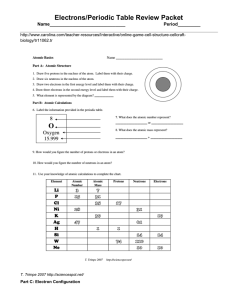
Electrons/Periodic Table Review Packet Name______________________________ Period_________
... Electrons/Periodic Table Review Packet Name______________________________ b. metals with unpredictable properties c. a halogen d. make good semiconductors e. alkali metals f. ...
... Electrons/Periodic Table Review Packet Name______________________________ b. metals with unpredictable properties c. a halogen d. make good semiconductors e. alkali metals f. ...
Unit 6 Review Packet - Old Saybrook Public Schools
... 3. Elements in Group 8A form the _________________. 6. The person in #8 ACROSS arranged the elements in order of increasing _______________ ________________ and similar properties. 8. _________________________ arranged the first usable Periodic Table in 1869. 9. As atomic numbers of the element ...
... 3. Elements in Group 8A form the _________________. 6. The person in #8 ACROSS arranged the elements in order of increasing _______________ ________________ and similar properties. 8. _________________________ arranged the first usable Periodic Table in 1869. 9. As atomic numbers of the element ...
Exam # 2 Review - HCC Learning Web
... Classify the following compounds : TiO2, H2O, NH3, HCl What is the chemical formula for the binary compound composed of Li+ and O2- ions? What is the chemical formula for the binary compound composed of Al3+ and O2- ions? What is the chemical formula for the binary compound composed of Sr2+ and Br- ...
... Classify the following compounds : TiO2, H2O, NH3, HCl What is the chemical formula for the binary compound composed of Li+ and O2- ions? What is the chemical formula for the binary compound composed of Al3+ and O2- ions? What is the chemical formula for the binary compound composed of Sr2+ and Br- ...
02 The structure of the periodic table II
... Mendeleev’s periodic table It made sense for iodine (I) to come after tellurium (Te) ...
... Mendeleev’s periodic table It made sense for iodine (I) to come after tellurium (Te) ...
Period 3 element
A period 3 element is one of the chemical elements in the third row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d orbital, but it is not filled until Period 4, such giving the period table its characteristic shape of ""two rows at a time"". All of the period 3 elements occur in nature and have at least one stable isotope.