Unit C3, C3.1
... John Newlands was one of the first chemists to arrange the known elements in order of increasing atomic mass. In 1866, he put forward the Law of Octaves. He suggested that there was a repeating pattern of elements with similar chemical properties every eighth element, just like the eighth note of an ...
... John Newlands was one of the first chemists to arrange the known elements in order of increasing atomic mass. In 1866, he put forward the Law of Octaves. He suggested that there was a repeating pattern of elements with similar chemical properties every eighth element, just like the eighth note of an ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
The Periodic Table Notes
... Fluorine, Chlorine, Bromine, and Iodine were all placed into the same group because they had similar properties and bonded with other elements in the same ratios ...
... Fluorine, Chlorine, Bromine, and Iodine were all placed into the same group because they had similar properties and bonded with other elements in the same ratios ...
C2- Topic 1: Atomic structure and the periodic table. Assessable
... Explain how Mendeleev: - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... Explain how Mendeleev: - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
Periodic Table Notes
... (Ca, Ba, Sr) were very similar. He also recognized that the atomic mass of Sr was about midway between that of Ca and Ba. He grouped these three elements together and called them a triad. ...
... (Ca, Ba, Sr) were very similar. He also recognized that the atomic mass of Sr was about midway between that of Ca and Ba. He grouped these three elements together and called them a triad. ...
C2 Topic 1 Can Do Sheet
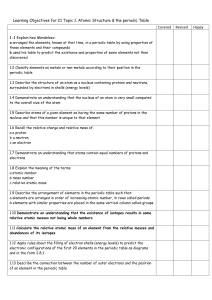
... Learning Objectives for C1 Topic 1. Atomic Structure & the periodic Table Covered 1.1 Explain how Mendeleev: a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some ele ...
... Learning Objectives for C1 Topic 1. Atomic Structure & the periodic Table Covered 1.1 Explain how Mendeleev: a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some ele ...
The History of the Modern Periodic Table
... (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. This quantity can only be the charge on the central positive nucleus.” ...
... (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. This quantity can only be the charge on the central positive nucleus.” ...
Mendeleev`s periodic table
... Describe how Mendeleev arranged the elements known at that time, in a Task periodic table by using properties of these elements and their compounds. Describe how Mendeleev used his table to predict the existence and properties ...
... Describe how Mendeleev arranged the elements known at that time, in a Task periodic table by using properties of these elements and their compounds. Describe how Mendeleev used his table to predict the existence and properties ...
The Periodic Law
... b. What is the mass of this atom in amus (to the nearest whole number)? c. Is this element Pt, Xe, I, or Bh? d. Identify two other elements that are in its group. 10. In a modern periodic table, every element is a member of both a horizontal row and a vertical column. Which one is the group, and whi ...
... b. What is the mass of this atom in amus (to the nearest whole number)? c. Is this element Pt, Xe, I, or Bh? d. Identify two other elements that are in its group. 10. In a modern periodic table, every element is a member of both a horizontal row and a vertical column. Which one is the group, and whi ...
Give the name and symbol for the element found in
... Metalloids have properties of both metals and nonmetals; they are usually semi-conductors of electricity 7. Who is Dmitri Mendeleev, and what was his contribution to chemistry? Dmitri Mendeleev was a Russian scientist credited with creating the first periodic table. He arranged all of the known elem ...
... Metalloids have properties of both metals and nonmetals; they are usually semi-conductors of electricity 7. Who is Dmitri Mendeleev, and what was his contribution to chemistry? Dmitri Mendeleev was a Russian scientist credited with creating the first periodic table. He arranged all of the known elem ...
The History of the Modern Periodic Table
... undiscovered elements. His most notable successes were with eka aluminium (= Gallium) and eka-silicon (= germanium). Lecoq de Boisbaudran discovered gallium in 1875 and reported its density as 4.7g cm -3, which did not agree with Mendeleev’s prediction of 5.9 g cm -3. ...
... undiscovered elements. His most notable successes were with eka aluminium (= Gallium) and eka-silicon (= germanium). Lecoq de Boisbaudran discovered gallium in 1875 and reported its density as 4.7g cm -3, which did not agree with Mendeleev’s prediction of 5.9 g cm -3. ...
The World of Chemistry - Mercer Island School District
... 1. What two types of properties are described in the video? 2. What are some examples of physical properties? 3. How many elements are on the modern periodic table? How many of these can be found in nature? 4. Why do the symbols for some elements (such as iron) seem to have no relationship to their ...
... 1. What two types of properties are described in the video? 2. What are some examples of physical properties? 3. How many elements are on the modern periodic table? How many of these can be found in nature? 4. Why do the symbols for some elements (such as iron) seem to have no relationship to their ...
u4_tqs - Teach-n-Learn-Chem
... Addison Wesley Chemistry by Michael S. Matta, Dennis D. Staley, A. Wilbraham, Edward L. Waterman ...
... Addison Wesley Chemistry by Michael S. Matta, Dennis D. Staley, A. Wilbraham, Edward L. Waterman ...
Periodic Table Timeline
... (Some of his “elements” were later determined to be compounds and included both heat and light.) ...
... (Some of his “elements” were later determined to be compounds and included both heat and light.) ...
Worksheet 2.1
... Match each element with the correct symbol by writing the number next to the correct symbol ...
... Match each element with the correct symbol by writing the number next to the correct symbol ...
Chemistry Study Guide - Atomic structure and the Periodic Table 2010
... 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: a. What is the structure of the atom and how are protons, neutrons, and electrons arran ...
... 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: a. What is the structure of the atom and how are protons, neutrons, and electrons arran ...
Page|1 - askIITians
... Q15. Mendeleev classified the elements on the basis of their ______. A. B. C. D. ...
... Q15. Mendeleev classified the elements on the basis of their ______. A. B. C. D. ...
PERIODIC TABLE - WordPress.com
... PERIODIC TABLE – open book worksheet Read pages 64-69 from your textbook [Chapter 3. Elements and Compounds, Section 3.1] and answer the following questions: 1. Which property of elements did Mendeleev use to arrange elements in his periodic table? 2. State three physical properties of metals. 3. Wh ...
... PERIODIC TABLE – open book worksheet Read pages 64-69 from your textbook [Chapter 3. Elements and Compounds, Section 3.1] and answer the following questions: 1. Which property of elements did Mendeleev use to arrange elements in his periodic table? 2. State three physical properties of metals. 3. Wh ...
PROFESSIONAL LEARNING COMMUNITY MODEL FOR ENTRY
... Dmitri Mendeleev, the Russian professor and chemist, is credited with conceptualizing the first periodic table. Mendeleev overcame sickness and strife in his youth to become a professor at Saint Petersburg State University. After becoming a teacher, he wrote the Principles of Chemistry (18681870). " ...
... Dmitri Mendeleev, the Russian professor and chemist, is credited with conceptualizing the first periodic table. Mendeleev overcame sickness and strife in his youth to become a professor at Saint Petersburg State University. After becoming a teacher, he wrote the Principles of Chemistry (18681870). " ...
Chapter 5 Reading Guide Please answer the following questions in
... At quick glance of the Mendeleev’s periodic table (bottom of page 127), what are some differences you spot from the modern periodic table (like the one on the wall or in your book)? ...
... At quick glance of the Mendeleev’s periodic table (bottom of page 127), what are some differences you spot from the modern periodic table (like the one on the wall or in your book)? ...
Unit 4 Review - Davis
... Electronegativity – attraction that one atom for the outer shell electron of another atom Ionization Energy – the energy needed to remove the outermost electron in an atom The atomic radius is a measure of the size of an atom. Modern Periodic Law – The properties of the elements are a periodic funct ...
... Electronegativity – attraction that one atom for the outer shell electron of another atom Ionization Energy – the energy needed to remove the outermost electron in an atom The atomic radius is a measure of the size of an atom. Modern Periodic Law – The properties of the elements are a periodic funct ...
The Periodic Table
... accurately determining the atomic masses of the elements. Russian chemist, Dmitri Mendeleev sought to arrange the elements using both the atomic masses and properties of each. • when the elements were arranged in order of increasing atomic mass, certain similarities occurred periodically. • his proc ...
... accurately determining the atomic masses of the elements. Russian chemist, Dmitri Mendeleev sought to arrange the elements using both the atomic masses and properties of each. • when the elements were arranged in order of increasing atomic mass, certain similarities occurred periodically. • his proc ...
The Periodic Table
... THE PERIODIC TABLE – PERIODS Periods: • Rows on the Periodic Table • Elements in the same period have the same number of electron shells (energy levels.) ...
... THE PERIODIC TABLE – PERIODS Periods: • Rows on the Periodic Table • Elements in the same period have the same number of electron shells (energy levels.) ...
Dmitri Mendeleev

Dmitri Ivanovich Mendeleev (/ˌmɛndəlˈeɪəf/; Russian: Дми́трий Ива́нович Менделе́ев; IPA: [ˈdmʲitrʲɪj ɪˈvanəvʲɪtɕ mʲɪndʲɪˈlʲejɪf]; 8 February 1834 – 2 February 1907 O.S. 27 January 1834 – 20 January 1907) was a Russian chemist and inventor. He formulated the Periodic Law, created his own version of the periodic table of elements, and used it to correct the properties of some already discovered elements and also to predict the properties of eight elements yet to be discovered.