* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Periodic Table
Alkali metal wikipedia , lookup
Group 12 element wikipedia , lookup
Boron group wikipedia , lookup
Livermorium wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Group 3 element wikipedia , lookup
Period 3 element wikipedia , lookup
Period 6 element wikipedia , lookup
Period 2 element wikipedia , lookup
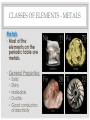
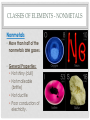
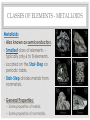
THE PERIODIC TABLE HISTORY • In 1869, Mendeleev studied the 63 known elements. • Mendeleev… • grouped elements with similar properties. • arranged elements by increasing atomic mass. • detected patterns that led him to develop the periodic table. • Periodic means having a regular, repeating pattern. Dmitri Mendeleev THE PERIODIC TABLE – PERIODS Periods: • Rows on the Periodic Table • Elements in the same period have the same number of electron shells (energy levels.) • • • • First Period – K shell. Second Period – K and L shells. Third Period – K, L, and M Shells. The periodic table includes 7 periods (largest atoms contain 7 electron shells – K, L, M, N, O, P, and Q). PERIODIC TABLE - GROUPS • Groups (or families) are columns on the periodic table. • Elements in the same group or family have similar properties. • Elements in the same group have the same number of electrons in their valence (outer) shell. CLASSES OF ELEMENTS - METALS Metals • Most of the elements on the periodic table are metals. • General Properties: • • • • • Solid Shiny Malleable Ductile Good conductors of electricity CLASSES OF ELEMENTS - NONMETALS Nonmetals • More than half of the nonmetals are gases. • General Properties: • Not shiny (dull) • Not malleable (brittle) • Not ductile • Poor conductors of electricity. CLASSES OF ELEMENTS - METALLOIDS Metalloids • Also known as semiconductors. • Smallest class of elements typically only 6 to 8 elements. • Located on the Stair-Step on periodic table. • Stair-Step divides metals from nonmetals. • General Properties: • Some properties of metals. • Some properties of nonmetals. READING THE PERIODIC TABLE • Atomic Number: number of protons in atom • Chemical Symbol: One or two letters, the first letter is capitalized • Background color: indicates metal, nonmetal, or metalloid • Color of symbol: indicates state (solid, liquid, gas) of element at room temperature • Atomic Mass: average mass number of all isotopes of the element. Metals – Blue, Nonmetals – Yellow, Metalloids - Green