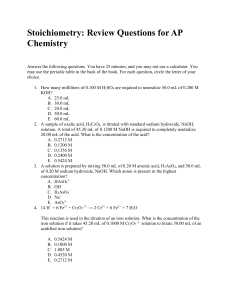
Quantity relationships: How much
... The calculated amount of product if is based on the assumption that all of the reactant is converted into product is called the theoretical yield. In laboratory or in industrial production, the actual amount of product isolated from a reaction is usually less than the theoretical yield, and it is ca ...
... The calculated amount of product if is based on the assumption that all of the reactant is converted into product is called the theoretical yield. In laboratory or in industrial production, the actual amount of product isolated from a reaction is usually less than the theoretical yield, and it is ca ...
Alternative Coverage of moles, molarity, and Chemical Calculations
... grams of methane, 32 grams of oxygen, and 48 grams of ozone must all contain the same number of molecules. In addition, because the atomic mass of titanium is equal to the molecular mass of ozone, the number of atoms in 48 grams of titanium must equal the number of molecules in 48 grams of ozone. We ...
... grams of methane, 32 grams of oxygen, and 48 grams of ozone must all contain the same number of molecules. In addition, because the atomic mass of titanium is equal to the molecular mass of ozone, the number of atoms in 48 grams of titanium must equal the number of molecules in 48 grams of ozone. We ...
Support Material
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
... Explain how vacancies are introduced in a solid NaCl crystal when divalent cations (M2+) are added to molten NaCl. ...
Stoichiometry
... This reaction is used in the titration of an iron solution. What is the concentration of the iron solution if it takes 45.20 mL of 0.1000 M Cr2O7 2– solution to titrate 50.00 mL of an acidified iron solution? A. B. C. D. E. ...
... This reaction is used in the titration of an iron solution. What is the concentration of the iron solution if it takes 45.20 mL of 0.1000 M Cr2O7 2– solution to titrate 50.00 mL of an acidified iron solution? A. B. C. D. E. ...
PART 3-ICHO 11-15
... first quantitatively separated from the precipitate, and then hydrogen sulphide was passed through the separated solution to saturation. The resulting precipitate containing metal B was separated, washed and dried. The mass of the precipitate was 0.6613 g. The precipitate containing the compounds of ...
... first quantitatively separated from the precipitate, and then hydrogen sulphide was passed through the separated solution to saturation. The resulting precipitate containing metal B was separated, washed and dried. The mass of the precipitate was 0.6613 g. The precipitate containing the compounds of ...
`bis (L-glutamine) potassium nitrate` crystal
... alkali metal nitrate does not result in any isomerisation / racemisation of the amino acid. The present study shows that use of nitrate salts of potassium (or sodium) in the medium does not inhibit the crystal growth of L-glutamine. Thus all these results add more credence to the above mentioned IR, ...
... alkali metal nitrate does not result in any isomerisation / racemisation of the amino acid. The present study shows that use of nitrate salts of potassium (or sodium) in the medium does not inhibit the crystal growth of L-glutamine. Thus all these results add more credence to the above mentioned IR, ...
A Dictionary of the New Chymical Nomenclature
... Lactat of alumine Lactat of ammoniac Lactat of antimony Lactat of arsenic Lactat of barytes Lactat of bismuth Lactat of cobalt Lactat of copper Lactat of gold Lactat of iron Lactat of lead Lactat of lime Lactat of magnesia Lactat of manganese Lactat of mercury Lactat of molybden Lactat of nickel [p. ...
... Lactat of alumine Lactat of ammoniac Lactat of antimony Lactat of arsenic Lactat of barytes Lactat of bismuth Lactat of cobalt Lactat of copper Lactat of gold Lactat of iron Lactat of lead Lactat of lime Lactat of magnesia Lactat of manganese Lactat of mercury Lactat of molybden Lactat of nickel [p. ...
equilibrium - eVirtualGuru
... varies with the experimental conditions such as concentrations of reactants, temperature, etc. Optimisation of the operational conditions is very important in industry and laboratory so that equilibrium is favorable in the direction of the desired product. Some important aspects of equilibrium invol ...
... varies with the experimental conditions such as concentrations of reactants, temperature, etc. Optimisation of the operational conditions is very important in industry and laboratory so that equilibrium is favorable in the direction of the desired product. Some important aspects of equilibrium invol ...
mod-5-revision-guide-4-transition-metals
... •Compounds with high oxidation states tend to be oxidising agents e.g MnO4•Compounds with low oxidation states are often reducing agents e.g V2+ & Fe2+ Reducing Chromium Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solu ...
... •Compounds with high oxidation states tend to be oxidising agents e.g MnO4•Compounds with low oxidation states are often reducing agents e.g V2+ & Fe2+ Reducing Chromium Cr3+ (green) and then Cr2+ (blue) are formed by reduction of Cr2O72- (orange) by the strong reducing agent zinc in (HCl) acid solu ...
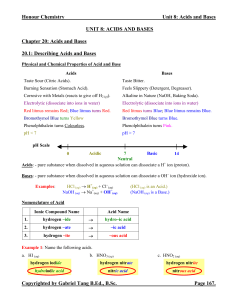
Unit 8 Acids and Bases Notes (answers)
... pH Scale: - a logarithmic scale to measure the acidity (relative [H3O+]) of a solution. - the lower the pH, the more acidic (less basic) is the solution (more [H3O+] and less [OH−]). - the higher the pH, the more basic (less acidic) is the solution (less [H3O+] and more [OH−]). - it is normally repo ...
... pH Scale: - a logarithmic scale to measure the acidity (relative [H3O+]) of a solution. - the lower the pH, the more acidic (less basic) is the solution (more [H3O+] and less [OH−]). - the higher the pH, the more basic (less acidic) is the solution (less [H3O+] and more [OH−]). - it is normally repo ...
Topic 6 Section C
... A drying agent may not necessarily be a dehydrating agent. For example, anhydrous calcium chloride can dry gases. However, it cannot remove chemically combined water from other compounds. ...
... A drying agent may not necessarily be a dehydrating agent. For example, anhydrous calcium chloride can dry gases. However, it cannot remove chemically combined water from other compounds. ...
Question Bank for Pre Board Exam(XII Chemistry)
... 2. Why urea has a sharp melting point but glass does not have? 3. A NaCl crystal is found to have Cs Cl structure. Guess how it might have happened? 4. Why is Frenkel defect not found in pure alkali metal halides? 5. NaCl and Cs Cl have similar formula. Then why they have different structures? 6. No ...
... 2. Why urea has a sharp melting point but glass does not have? 3. A NaCl crystal is found to have Cs Cl structure. Guess how it might have happened? 4. Why is Frenkel defect not found in pure alkali metal halides? 5. NaCl and Cs Cl have similar formula. Then why they have different structures? 6. No ...
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
... This revision introduces many new experiments and revises others in an attempt to keep abreast of the rapid developments in physical chemistry. Some of the former experiments have been eliminated or expanded because they have found their way into earlier courses and are already known to students, wh ...
Chem13-14PrecipABNeut
... with extensive memory during tests. If a type of calculator is specified for your course, buy two if possible. When one becomes broken or lost, you will have a familiar backup if the bookstore is sold out later in the term. If no type of calculator is specified for your course, any inexpensive calcu ...
... with extensive memory during tests. If a type of calculator is specified for your course, buy two if possible. When one becomes broken or lost, you will have a familiar backup if the bookstore is sold out later in the term. If no type of calculator is specified for your course, any inexpensive calcu ...
SQA Advanced Higher Chemistry Unit 2 Principles of Chemical
... Which of the following statements applies to this equation? 1. Calcium carbonate reacts with hydrochloric acid to produce calcium chloride solution, water and carbon dioxide. 2. One formula unit of calcium carbonate reacts with two formula units of hydrochloric acid to produce one formula unit each ...
... Which of the following statements applies to this equation? 1. Calcium carbonate reacts with hydrochloric acid to produce calcium chloride solution, water and carbon dioxide. 2. One formula unit of calcium carbonate reacts with two formula units of hydrochloric acid to produce one formula unit each ...
X: Ag, Ca, In, Li, Na, Sn, Sr and Zn
... additions of Li, Na, Ca, Zn, Ag, In, Sr, and Sn can improve the mechanical properties of Mgbased alloys, by forming secondary precipitates in the Mg matrix. In developing new magnesium alloys, it is important to understand their constitution (microstructure) and thermodynamic behaviour. Obtaining su ...
... additions of Li, Na, Ca, Zn, Ag, In, Sr, and Sn can improve the mechanical properties of Mgbased alloys, by forming secondary precipitates in the Mg matrix. In developing new magnesium alloys, it is important to understand their constitution (microstructure) and thermodynamic behaviour. Obtaining su ...
CHAPTER 18
... CHECK FOR UNDERSTANDING Explain The two terms dynamic and equilibrium seem to contradict each other at first. Dynamic means that changes are occurring, but equilibrium means that no overall change is happening. Given this, explain why the combination of the two, dynamic equilibrium, is correct. ...
... CHECK FOR UNDERSTANDING Explain The two terms dynamic and equilibrium seem to contradict each other at first. Dynamic means that changes are occurring, but equilibrium means that no overall change is happening. Given this, explain why the combination of the two, dynamic equilibrium, is correct. ...
Liquid–liquid extraction

Liquid–liquid extraction (LLE) consists in transferring one (or more) solute(s) contained in a feed solution to another immiscible liquid (solvent). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called raffinate.Liquid–liquid extraction also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid into another liquid phase. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment. This type of process is commonly performed after a chemical reaction as part of the work-up.The term partitioning is commonly used to refer to the underlying chemical and physical processes involved in liquid–liquid extraction, but on another reading may be fully synonymous with it. The term solvent extraction can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.Solvent extraction is used in nuclear reprocessing, ore processing, the production of fine organic compounds, the processing of perfumes, the production of vegetable oils and biodiesel, and other industries.Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salts, metals can be extracted from one phase to the other. This is related to a mercury electrode where a metal can be reduced, the metal will often then dissolve in the mercury to form an amalgam that modifies its electrochemistry greatly. For example, it is possible for sodium cations to be reduced at a mercury cathode to form sodium amalgam, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A detergent or fine solid can be used to stabilize an emulsion, or third phase.