Chemistry 111: Exam 1 Topics
... pressure/temp/volume based on molecular collisions Understand how to read a barometer Understand how to read a manometer Be able to convert pressure units Remember to use Kelvin! Understand what’s held constant in Boyle’s Law, Charles’ Law Use combined gas law (P1V1/T1=P2V2/T2 is given). ...
... pressure/temp/volume based on molecular collisions Understand how to read a barometer Understand how to read a manometer Be able to convert pressure units Remember to use Kelvin! Understand what’s held constant in Boyle’s Law, Charles’ Law Use combined gas law (P1V1/T1=P2V2/T2 is given). ...
Historical burdens on physics 77 Names of the ideal gas law
... of the ideal gas, and others. Since the equation relates more than two variables, one may be interested in the relationship between only two of these quantities, keeping the remaining variables constant. The corresponding relations are known under particular names. The relation between p and V is Bo ...
... of the ideal gas, and others. Since the equation relates more than two variables, one may be interested in the relationship between only two of these quantities, keeping the remaining variables constant. The corresponding relations are known under particular names. The relation between p and V is Bo ...
File 3 - College of Science | Oregon State University
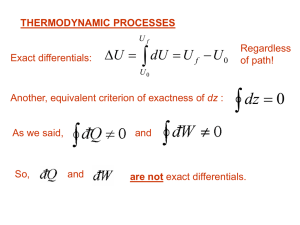
... of those coefficients are given in many books. We have already discussed the heat capacities Cv and Cp that are such “response functions”. Let’s introduce more: ...
... of those coefficients are given in many books. We have already discussed the heat capacities Cv and Cp that are such “response functions”. Let’s introduce more: ...