CH221 CLASS 13
... the oxymercuration and hydroboration /oxidation procedures. Orientation (regioselectivity) and stereoselectivity are discussed for each addition reaction and are closely compared for the two kinds of hydration procedures. Introduction Electrophilic addition reactions of alkenes are useful, making po ...
... the oxymercuration and hydroboration /oxidation procedures. Orientation (regioselectivity) and stereoselectivity are discussed for each addition reaction and are closely compared for the two kinds of hydration procedures. Introduction Electrophilic addition reactions of alkenes are useful, making po ...
Organometallic Methods for Forming and Cleaving Carbon
... Carbohydrates with protecting groups on all alcohol groups except the primary alcohol were prepared and subjected to the iridium catalyzed dehydrogenative decarbonylation reaction where primary alcohols are converted into the corresponding one carbon shorter products. Modest conversions were obtaine ...
... Carbohydrates with protecting groups on all alcohol groups except the primary alcohol were prepared and subjected to the iridium catalyzed dehydrogenative decarbonylation reaction where primary alcohols are converted into the corresponding one carbon shorter products. Modest conversions were obtaine ...
university of london thesis
... in this area, it is still difficult to make product predictions with confidence. Some processes are clean and occur in high yields,14 whilst many others give low yields and multicomponent mixture o f products.15 These products range from simple hydride shift to carbonyl isomers, through to structure ...
... in this area, it is still difficult to make product predictions with confidence. Some processes are clean and occur in high yields,14 whilst many others give low yields and multicomponent mixture o f products.15 These products range from simple hydride shift to carbonyl isomers, through to structure ...
Improved Synthesis, Separation, Transition Metal Coordination and
... P{1H} spectra of meso-Ni2Cl4(et,ph-P4) with 1-hexene in acetone-d6/D2O recorded at 15°C (light blue), 10°C (dark blue), 25°C (black), 50°C (orange), 80°C (purple), and 100°C (red). For higher temperatures the NMR tube was tube pressurized to 90 psi with O2 ......................................... ...
... P{1H} spectra of meso-Ni2Cl4(et,ph-P4) with 1-hexene in acetone-d6/D2O recorded at 15°C (light blue), 10°C (dark blue), 25°C (black), 50°C (orange), 80°C (purple), and 100°C (red). For higher temperatures the NMR tube was tube pressurized to 90 psi with O2 ......................................... ...
Stereoselective Construction of a β
... 1018 or (S,S)-bis(oxazoline) 1119) at -78 °C. The use of BuLi and 10 in THF resulted in poor enantioselectivity (entries 1 and 3), while in hexane the reactions gave none of the [2,3] Wittig rearrangement products (entries 2 and 4). Interestingly, the combination of t-BuLi and 11 in hexane was found ...
... 1018 or (S,S)-bis(oxazoline) 1119) at -78 °C. The use of BuLi and 10 in THF resulted in poor enantioselectivity (entries 1 and 3), while in hexane the reactions gave none of the [2,3] Wittig rearrangement products (entries 2 and 4). Interestingly, the combination of t-BuLi and 11 in hexane was found ...
New Applications for Sulfur-Based Leaving Groups in Synthesis
... reaction of an aliphatic potassium alkoxide with an aromatic alkynyl sulfonamide. The mechanism of this process has been explored via a combination of synthetic chemistry and electron paramagnetic resonance spectroscopy (EPR) and the findings of these experiments will be discussed. The synthesis of ...
... reaction of an aliphatic potassium alkoxide with an aromatic alkynyl sulfonamide. The mechanism of this process has been explored via a combination of synthetic chemistry and electron paramagnetic resonance spectroscopy (EPR) and the findings of these experiments will be discussed. The synthesis of ...
Electrophilic Selenium Catalysis with Electrophilic N
... of N-chlorosuccinimide (NCS). Subsequent chloroselenenylation–deselenenylation process afforded allylic chloride and regenerated PhSeCl. After this seminal work, several oxidative systems such as PhSeSePh/persulfate [12–19], PhSeSePh/H2 O2 [20–25], PhSeSePh/hypervalent iodide [26–29] and so on [30–3 ...
... of N-chlorosuccinimide (NCS). Subsequent chloroselenenylation–deselenenylation process afforded allylic chloride and regenerated PhSeCl. After this seminal work, several oxidative systems such as PhSeSePh/persulfate [12–19], PhSeSePh/H2 O2 [20–25], PhSeSePh/hypervalent iodide [26–29] and so on [30–3 ...
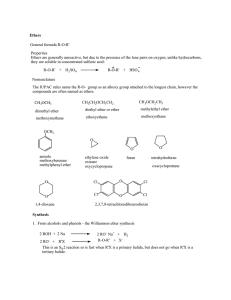
Ethers General formula R-O-R` Properties Ethers are generally
... CH3 Hg(OCOCH 3)2/CH3 CH2OH H2C C CH 3 H2C C CH 3 Hg OCH2 CH3 O C CH 3 ...
... CH3 Hg(OCOCH 3)2/CH3 CH2OH H2C C CH 3 H2C C CH 3 Hg OCH2 CH3 O C CH 3 ...
PowerPoint 演示文稿
... in his early work, to test the theory. Studies of aliphatic substitutions and eliminations, often with his long-time collaborator E. D. Hughes, led to I ncorporation into the standard language of chemistry of such words as nucleophile, electrophile, inductive and mesomeric (resonance) effects, and s ...
... in his early work, to test the theory. Studies of aliphatic substitutions and eliminations, often with his long-time collaborator E. D. Hughes, led to I ncorporation into the standard language of chemistry of such words as nucleophile, electrophile, inductive and mesomeric (resonance) effects, and s ...
Recent advances in homogeneous nickel catalysis
... (Fig. 1) is needed. Nickel is a relatively electropositive late transition metal. Therefore, oxidative addition5, which results in loss of electron density around nickel, tends to occur quite readily (though, conversely, reductive elimination is correspondingly more difficult)6. This facile oxidativ ...
... (Fig. 1) is needed. Nickel is a relatively electropositive late transition metal. Therefore, oxidative addition5, which results in loss of electron density around nickel, tends to occur quite readily (though, conversely, reductive elimination is correspondingly more difficult)6. This facile oxidativ ...
Alcohols
... –SH bonded to an aromatic ring • Enols and enethiols are compounds with the –OH or –SH bonded to a vinylic, sp2-hybridized carbon ...
... –SH bonded to an aromatic ring • Enols and enethiols are compounds with the –OH or –SH bonded to a vinylic, sp2-hybridized carbon ...
Alkyl Halides02
... This is also a qualitative test for identifying alcohols, i.e, the Lucas test. HCl with ZnCl 2 catalyst are used. 3 alcohols react quickly, 2 slowly, and 1 don’t react. Note that 1 and 2 alcohols will react with special reagents to produce alkyl halides (i.e., thionyl chloride, SOCl2, or PBr3) ...
... This is also a qualitative test for identifying alcohols, i.e, the Lucas test. HCl with ZnCl 2 catalyst are used. 3 alcohols react quickly, 2 slowly, and 1 don’t react. Note that 1 and 2 alcohols will react with special reagents to produce alkyl halides (i.e., thionyl chloride, SOCl2, or PBr3) ...
HIGHLY SELECTIVE RHODIUM–CATALYZED C–H BORYLATIONS IN
... organoboron starting materials. C–H borylation (activation) has provided an interesting approach to alleviate the requirement for prefunctionalized molecules such as aryl halides to obtain these desired organoboron substrates. We report the first use of a rhodium N–heterocyclic carbene (NHC) complex ...
... organoboron starting materials. C–H borylation (activation) has provided an interesting approach to alleviate the requirement for prefunctionalized molecules such as aryl halides to obtain these desired organoboron substrates. We report the first use of a rhodium N–heterocyclic carbene (NHC) complex ...
Grignard Reagents brochure
... react with a broad range of electrophilic substrates. The reactions with aldehydes, ketones27, esters, acids49 and acid chlorides is one of the most useful reaction in organic chemistry for the formation of C-C-bonds50,51,52. The reaction has a very broad scope, and the Grignard reagent can be aliph ...
... react with a broad range of electrophilic substrates. The reactions with aldehydes, ketones27, esters, acids49 and acid chlorides is one of the most useful reaction in organic chemistry for the formation of C-C-bonds50,51,52. The reaction has a very broad scope, and the Grignard reagent can be aliph ...
Melt Modification of Poly(styrene-co-maleic anhydride)
... esterification to the product side by removing the in situ generated carboxylic acid from the system by a subsequent reaction. For example, 1,3-oxazolines are well known to afford quantitative conversion of carboxylic acids both in melt and in solution processes.16 Because of their high reactivity, ...
... esterification to the product side by removing the in situ generated carboxylic acid from the system by a subsequent reaction. For example, 1,3-oxazolines are well known to afford quantitative conversion of carboxylic acids both in melt and in solution processes.16 Because of their high reactivity, ...
Chapter Seven PPT
... • E1 Elimination Reactions • Zaitsev’s Rule • Carbocation Rearrangement • Dehydration and Dehydrohalogenation Reactions • Synthesis of Alkynes • Hydrogenation Reactions (Alkynes to E/Z Alkenes) • Unsaturation Numbers; Utility in Structure Determination ...
... • E1 Elimination Reactions • Zaitsev’s Rule • Carbocation Rearrangement • Dehydration and Dehydrohalogenation Reactions • Synthesis of Alkynes • Hydrogenation Reactions (Alkynes to E/Z Alkenes) • Unsaturation Numbers; Utility in Structure Determination ...
synthetic approaches for quinoline and isoquinoline
... of a benzene ring fused to a pyridine ring5 . In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives.1‐Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine and morphine. The isoquino ...
... of a benzene ring fused to a pyridine ring5 . In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives.1‐Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine and morphine. The isoquino ...
Alkyl Halides SN and E reactions
... 3. Consider the nature of the solvent: For SN1 reactions, the solvent affects the rate only if it influences the stability of the charged transition state, i.e., the C+. The Nu:- is not involved in the rate determining step so solvent effects on the Nu:- do not affect the rate of SN1 reactions. ...
... 3. Consider the nature of the solvent: For SN1 reactions, the solvent affects the rate only if it influences the stability of the charged transition state, i.e., the C+. The Nu:- is not involved in the rate determining step so solvent effects on the Nu:- do not affect the rate of SN1 reactions. ...
Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... 3. Consider the nature of the solvent: For SN1 reactions, the solvent affects the rate only if it influences the stability of the charged transition state, i.e., the C+. The Nu:- is not involved in the rate determining step so solvent effects on the Nu:- do not affect the rate of SN1 reactions. ...
... 3. Consider the nature of the solvent: For SN1 reactions, the solvent affects the rate only if it influences the stability of the charged transition state, i.e., the C+. The Nu:- is not involved in the rate determining step so solvent effects on the Nu:- do not affect the rate of SN1 reactions. ...
Reactions of Alkyl Halides (SN1, SN2, E1, and E2 reactions)
... 3. Consider the nature of the solvent: For SN1 reactions, the solvent affects the rate only if it influences the stability of the charged transition state, i.e., the C+. The Nu:- is not involved in the rate determining step so solvent effects on the Nu:- do not affect the rate of SN1 reactions. ...
... 3. Consider the nature of the solvent: For SN1 reactions, the solvent affects the rate only if it influences the stability of the charged transition state, i.e., the C+. The Nu:- is not involved in the rate determining step so solvent effects on the Nu:- do not affect the rate of SN1 reactions. ...
Chemistry 162 Workbook 10.6
... environment when taking these exams. Many students unfortunately suffer from test anxiety and much of this anxiety can be avoided by increased knowledge of material, as well as experience ...
... environment when taking these exams. Many students unfortunately suffer from test anxiety and much of this anxiety can be avoided by increased knowledge of material, as well as experience ...
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
Chloroperbenzoic_aci..
... TFPAA is used for unreactive and heat-sensitive substrates; its reactivity permits the use of low reaction temperatures. The recently introduced reagent magnesium monoperphthalate (MMPP) (see Monoperoxyphthalic Acid) is more stable than m-CPBA and has many applications.4 Epoxidations of hydroxyalken ...
... TFPAA is used for unreactive and heat-sensitive substrates; its reactivity permits the use of low reaction temperatures. The recently introduced reagent magnesium monoperphthalate (MMPP) (see Monoperoxyphthalic Acid) is more stable than m-CPBA and has many applications.4 Epoxidations of hydroxyalken ...
Diels–Alder reaction
.png?width=300)
The Diels–Alder reaction is an organic chemical reaction (specifically, a [4+2] cycloaddition) between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. It was first described by Otto Paul Hermann Diels and Kurt Alder in 1928, for which work they were awarded the Nobel Prize in Chemistry in 1950. The Diels–Alder reaction is particularly useful in synthetic organic chemistry as a reliable method for forming 6-membered systems with good control over regio- and stereochemical properties. The underlying concept has also been applied to other π-systems, such as carbonyls and imines, to furnish the corresponding heterocycles, known as the hetero-Diels–Alder reaction. Diels–Alder reactions can be reversible under certain conditions; the reverse reaction is known as the retro-Diels–Alder reaction.