Chem 30BL_Lecture 2_.. - UCLA Chemistry and Biochemistry
... • Regiocontrol is observed due to pre-existing double bonds 33% H 2SO4 ...
... • Regiocontrol is observed due to pre-existing double bonds 33% H 2SO4 ...
Chem 30BL * Lecture 2 - UCLA Chemistry and Biochemistry
... • Regiocontrol is observed due to pre-existing double bonds 33% H2SO4 ...
... • Regiocontrol is observed due to pre-existing double bonds 33% H2SO4 ...
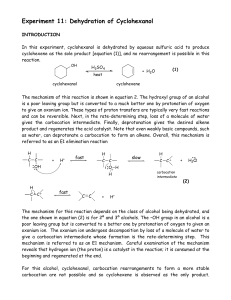
Dehydration of Cyclohexanol
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
ENZYMES - SELF STUDY QUESTIONS 1. A chemical reaction has a
... 1. A chemical reaction has a ∆ Go = -2000 kJ/mol. If this were an enzyme-catalyzed reaction what can you predict about the kinetics? a. It will exhibit very rapid kinetics b. It will exhibit very slow kinetics c. The kinetics of the reaction can not be predicted d. The kinetics depend on the nature ...
... 1. A chemical reaction has a ∆ Go = -2000 kJ/mol. If this were an enzyme-catalyzed reaction what can you predict about the kinetics? a. It will exhibit very rapid kinetics b. It will exhibit very slow kinetics c. The kinetics of the reaction can not be predicted d. The kinetics depend on the nature ...
Biochemistry 462a - Enzymes Extra Questions
... 1. A chemical reaction has a Go = -2000 kJ/mol. If this were an enzyme-catalyzed reaction what can you predict about the kinetics? a. It will exhibit very rapid kinetics b. It will exhibit very slow kinetics c. The kinetics of the reaction can not be predicted d. The kinetics depend on the nature ...
... 1. A chemical reaction has a Go = -2000 kJ/mol. If this were an enzyme-catalyzed reaction what can you predict about the kinetics? a. It will exhibit very rapid kinetics b. It will exhibit very slow kinetics c. The kinetics of the reaction can not be predicted d. The kinetics depend on the nature ...
(substituted) carbon
... Hydroboration-oxidation of alkenes allows stereospecific and regioselective synthesis of alcohols. The reaction sequence exhibits anti-Markovnikov regioselectivity which complements acid-catalyzed hydration and oxymercurationdemercuration. The reaction mechanism does not involve a carbocation and t ...
... Hydroboration-oxidation of alkenes allows stereospecific and regioselective synthesis of alcohols. The reaction sequence exhibits anti-Markovnikov regioselectivity which complements acid-catalyzed hydration and oxymercurationdemercuration. The reaction mechanism does not involve a carbocation and t ...
DMC (double metal cyanide) catalyst DMC catalyst is used
... molecular weight polether polyols. In conventional base catalyzed oxyalkylation reaction, propylene oxide and certain other alkylene oxides are subject to a competing internal rearrangement that generates unsaturated alcohols. The resulting products will contain allyl alcohol initiated, monofunction ...
... molecular weight polether polyols. In conventional base catalyzed oxyalkylation reaction, propylene oxide and certain other alkylene oxides are subject to a competing internal rearrangement that generates unsaturated alcohols. The resulting products will contain allyl alcohol initiated, monofunction ...
Nucleophilic Substitution Reactions of Epoxides
... aromatic ring has been converted into an epoxide. What happens to aromatic compounds when they enter the body as a foreign substance (such as cigarette smoke, drugs, charcoalbroiled meats or automobile exhaust)? ...
... aromatic ring has been converted into an epoxide. What happens to aromatic compounds when they enter the body as a foreign substance (such as cigarette smoke, drugs, charcoalbroiled meats or automobile exhaust)? ...
CH 3 Br + Nu
... 10. Which statement(s) is/are true of an E1 elimination? A) it is a two-step process and has the same first step as a SN1 mechanism B) it involves the formation of the carbocation from elimination of a good leaving group C) a common competing reaction is rearrangement of a less stable carbocation t ...
... 10. Which statement(s) is/are true of an E1 elimination? A) it is a two-step process and has the same first step as a SN1 mechanism B) it involves the formation of the carbocation from elimination of a good leaving group C) a common competing reaction is rearrangement of a less stable carbocation t ...
الشريحة 1
... A pi bond is one in which the electrons in the p orbitals are held above and below the plane of the molecule. The sigma bond is stronger than the pi bond. A double bond is formed from a sigma bond and a pi bond, and so it is stronger than a single bond. ...
... A pi bond is one in which the electrons in the p orbitals are held above and below the plane of the molecule. The sigma bond is stronger than the pi bond. A double bond is formed from a sigma bond and a pi bond, and so it is stronger than a single bond. ...
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015
... Formal product of the addition is the enol, which is often not isolated ...
... Formal product of the addition is the enol, which is often not isolated ...
Chapter 7
... • Rearrangements will always occur when an alkyl group or a hydrogen can shift to form a more stable carbocation!! • 1,2-methyl shift • 1,2-hydride shift • Remember, these shifts occur to increase stability so other forms of stability must be considered as well! ...
... • Rearrangements will always occur when an alkyl group or a hydrogen can shift to form a more stable carbocation!! • 1,2-methyl shift • 1,2-hydride shift • Remember, these shifts occur to increase stability so other forms of stability must be considered as well! ...
Chemistry 3719L – Week 9 Reduction of Benzil with Sodium
... • Filter the solid, dry for 15 minutes then weigh the product and obtain the melting point. • If possible you should obtain an IR spectrum of your product to compare with that of the benzil starting material. This information will be used in your report to prove that the functional group conversion ...
... • Filter the solid, dry for 15 minutes then weigh the product and obtain the melting point. • If possible you should obtain an IR spectrum of your product to compare with that of the benzil starting material. This information will be used in your report to prove that the functional group conversion ...
Lecture Resource ()
... Irene Lee Case Western Reserve University Cleveland, OH ©2004, Prentice Hall ...
... Irene Lee Case Western Reserve University Cleveland, OH ©2004, Prentice Hall ...
Chapter 7 - Alkenes and Alkynes I less substituted alkene due to
... How to Favor E2: - Reaction conditions that favor elimination by E1 should be avoided due to the highly competitive SN 1 mechanism - To favor E2, a secondary or tertiary alkyl halide should be used - If there is only a possibility for a primary alkyl halide, use a bulky base - Use a higher concentra ...
... How to Favor E2: - Reaction conditions that favor elimination by E1 should be avoided due to the highly competitive SN 1 mechanism - To favor E2, a secondary or tertiary alkyl halide should be used - If there is only a possibility for a primary alkyl halide, use a bulky base - Use a higher concentra ...
CHE-06 year 2004
... CH3CH2CH2Br + CH3OH CH3CH2CH2OCH3 + HBr or CH3CH2CH2Br + CH3O¯ CH3CH2CH2OCH3 +Br¯ ...
... CH3CH2CH2Br + CH3OH CH3CH2CH2OCH3 + HBr or CH3CH2CH2Br + CH3O¯ CH3CH2CH2OCH3 +Br¯ ...
Solution Key - Chemistry With BT
... Is the stereoisomer obtained in the reaction above optically active? Explain. No, it is not possible to obtain a chiral product from an achiral reactant unless chiral reaction conditions are utilized, such as enzyme catalysis ...
... Is the stereoisomer obtained in the reaction above optically active? Explain. No, it is not possible to obtain a chiral product from an achiral reactant unless chiral reaction conditions are utilized, such as enzyme catalysis ...
Group B_reaction of alkenes
... groups, with its pair of electrons shifts to the adjacent +vely charged C to form a stable tertiary carbocation. • 1,2-methyl shift •Major product- is the most stable carbocation. ...
... groups, with its pair of electrons shifts to the adjacent +vely charged C to form a stable tertiary carbocation. • 1,2-methyl shift •Major product- is the most stable carbocation. ...
A Diels-Alder Synthesis
... The Diels-Alder reaction usually proceeds with endo selectivity. This means that the product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes mo ...
... The Diels-Alder reaction usually proceeds with endo selectivity. This means that the product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes mo ...
Exam 2 SOLUTION
... a) How many steps are in the mechanism to this reaction? There are 4 steps to this mechanism. b) Which step is the rate-determining step? The second step, with the highest activation barrier, is the RDS. c) Label ∆Hrxn and the activation energies on the graph. 4. Using curved-arrow notation, give th ...
... a) How many steps are in the mechanism to this reaction? There are 4 steps to this mechanism. b) Which step is the rate-determining step? The second step, with the highest activation barrier, is the RDS. c) Label ∆Hrxn and the activation energies on the graph. 4. Using curved-arrow notation, give th ...
Here is the Original File - University of New Hampshire
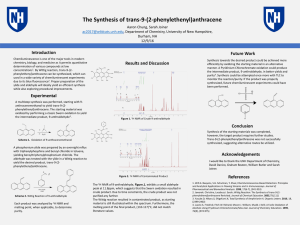
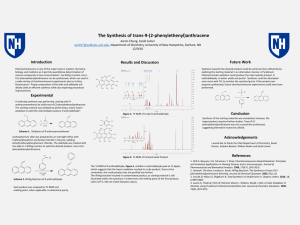
... chemistry, biology, and medicine as it permits quantitative determination of various compounds at low concentrations1. By Wittig reaction, trans-9-(2phenylethenyl)anthracene can be synthesized, which can used in a wide variety of chemiluminescent experiments due to its blue fluorescence2. Proper pre ...
... chemistry, biology, and medicine as it permits quantitative determination of various compounds at low concentrations1. By Wittig reaction, trans-9-(2phenylethenyl)anthracene can be synthesized, which can used in a wide variety of chemiluminescent experiments due to its blue fluorescence2. Proper pre ...
The Synthesis of trans-9-(2
... [email protected], Department of Chemistry, University of New Hampshire, Durham, NH ...
... [email protected], Department of Chemistry, University of New Hampshire, Durham, NH ...
Chapter 7
... • Rearrangements will always occur when an alkyl group or a hydrogen can shift to form a more stable carbocation!! • 1,2-methyl shift • 1,2-hydride shift • Remember, these shifts occur to increase stability so other forms of stability must be considered as well! ...
... • Rearrangements will always occur when an alkyl group or a hydrogen can shift to form a more stable carbocation!! • 1,2-methyl shift • 1,2-hydride shift • Remember, these shifts occur to increase stability so other forms of stability must be considered as well! ...
Calculating Percent Yield
... Organic reactions typically do not give 100% yields, meaning all of the starting material does not get converted to the product. The percent of starting material that is converted to product in a chemical reaction is referred to as the percent yield. The percent yield can be calculated if the follow ...
... Organic reactions typically do not give 100% yields, meaning all of the starting material does not get converted to the product. The percent of starting material that is converted to product in a chemical reaction is referred to as the percent yield. The percent yield can be calculated if the follow ...
Total marks available
... This is a question about halogenoalkanes. (a) Halogenoalkanes can react with hydroxide ions in different ways depending on the conditions used. Using 1-chloro-1-fluoroethane, CH3CHClF, as an example of a halogenoalkane, the following reaction could occur in aqueous solution. CH3CHClF + OH− → CH3CHOH ...
... This is a question about halogenoalkanes. (a) Halogenoalkanes can react with hydroxide ions in different ways depending on the conditions used. Using 1-chloro-1-fluoroethane, CH3CHClF, as an example of a halogenoalkane, the following reaction could occur in aqueous solution. CH3CHClF + OH− → CH3CHOH ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.