... 6. How will you prepare phenyl methyl ether from phenol using Williamson’s synthesis? 7. What happens when calcium acetate is heated? Give its equation. 8. What is Norrish type –I reaction. 9. What is trans esterification. 10. Arrange the following in terms of increasing acid strength and give reaso ...
Oxidative Addition
... such as H2 or CH3‐I. A−B bond is broken, and M−A and M−B bonds are formed. ...
... such as H2 or CH3‐I. A−B bond is broken, and M−A and M−B bonds are formed. ...
Organic Chemistry 1 1st Hour Exam Student ID # Name
... (b) Explain why one product is the major isomer based on their reaction coordinate diagrams that show the two different reaction progresses (or pathways) to give the two different products, the major and the minor products. Explain the reaction results using the Hammond postulate. ...
... (b) Explain why one product is the major isomer based on their reaction coordinate diagrams that show the two different reaction progresses (or pathways) to give the two different products, the major and the minor products. Explain the reaction results using the Hammond postulate. ...
Eliminations
... So far we have discussed only one reaction type: substitutions. We now introduce a new reaction type: eliminations. They are related to substitution reactions and often accompany them. Instead of substituting ...
... So far we have discussed only one reaction type: substitutions. We now introduce a new reaction type: eliminations. They are related to substitution reactions and often accompany them. Instead of substituting ...
File
... Deduce a reaction pathway for the two-stage conversion of 1-bromopropane to 1-butylamine (butan-1-amine). Your answer should include an equation for each stage of the reaction and the reaction conditions for the second stage. ...
... Deduce a reaction pathway for the two-stage conversion of 1-bromopropane to 1-butylamine (butan-1-amine). Your answer should include an equation for each stage of the reaction and the reaction conditions for the second stage. ...
Organic Reactions
... ∆G is negative, energy is released, exergonic reaction ∆G is positive, energy absorbed, endergonic reaction ...
... ∆G is negative, energy is released, exergonic reaction ∆G is positive, energy absorbed, endergonic reaction ...
Chapter 7
... • Rearrangements will always occur when an alkyl group or a hydrogen can shift to form a more stable carbocation!! • 1,2-methyl shift • 1,2-hydride shift • Remember, these shifts occur to increase stability so other forms of stability must be considered as well! ...
... • Rearrangements will always occur when an alkyl group or a hydrogen can shift to form a more stable carbocation!! • 1,2-methyl shift • 1,2-hydride shift • Remember, these shifts occur to increase stability so other forms of stability must be considered as well! ...
I (21 points) Complete the following reactions by providing starting
... A. (JOC, 2008, ASAP, Loh) Chemists have been studying the Barbier-Grignard reactions with the goal of affecting the carbon-carbon bond forming reaction in solvents like water. Recent developments include the use of indium metal catalysts that react through single electron transfer mechanisms. Show t ...
... A. (JOC, 2008, ASAP, Loh) Chemists have been studying the Barbier-Grignard reactions with the goal of affecting the carbon-carbon bond forming reaction in solvents like water. Recent developments include the use of indium metal catalysts that react through single electron transfer mechanisms. Show t ...
Chemistry: Selected Topics
... Final competences 1 Understanding the relation between reaction rate and reaction mechanism 2 Being able to develop the rate equation of a chemical reaction 3 Knowledge of the properties and synthesis of important types of inorganic polymers 4 Knowledge of the relation between chemical structure ...
... Final competences 1 Understanding the relation between reaction rate and reaction mechanism 2 Being able to develop the rate equation of a chemical reaction 3 Knowledge of the properties and synthesis of important types of inorganic polymers 4 Knowledge of the relation between chemical structure ...
Exp 19 - Diphenylacetylene_2015
... *If you don’t have enough meso-stilbene dibromide from part 1, you can obtain some from the reagent hood. If you have enough, however, you should use the material that you prepared. Weigh approximately 150 mg of the meso-stilbene dibromide, prepared in part 1, into a 5-mL conical reaction vial. Next ...
... *If you don’t have enough meso-stilbene dibromide from part 1, you can obtain some from the reagent hood. If you have enough, however, you should use the material that you prepared. Weigh approximately 150 mg of the meso-stilbene dibromide, prepared in part 1, into a 5-mL conical reaction vial. Next ...
PowerPoint **
... Carbonyl Group Under basic conditions, carbonyl compounds are electrophilic at carbonyl C and nuclephilic at α C’s. ...
... Carbonyl Group Under basic conditions, carbonyl compounds are electrophilic at carbonyl C and nuclephilic at α C’s. ...
Document
... The formation of carbon-carbon bonds is one of the most widely studied areas in organic synthesis. One class of carbon-carbon bond forming reactions involves the nucleophilic addition of vinyl or allyl organometallics to aldhydes, yielding allylic or homoallylic alcohols. The stereochemical unpredic ...
... The formation of carbon-carbon bonds is one of the most widely studied areas in organic synthesis. One class of carbon-carbon bond forming reactions involves the nucleophilic addition of vinyl or allyl organometallics to aldhydes, yielding allylic or homoallylic alcohols. The stereochemical unpredic ...
Applications of Phosphorus, Sulfur, Silicon and Boron Chemistry:
... Formulate the product arising from oxidation, protonolysis, halogenation or amination of an alkyl- or alkenylborane. ...
... Formulate the product arising from oxidation, protonolysis, halogenation or amination of an alkyl- or alkenylborane. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 18. Explain the mechanism of markownikoff and antimarkownikoff addition of propene. 19. Write short notes on a)hydroboration reaction b) addition polymerization reaction 20. Explain the mode of hybridization of carbon in methane, ethylene and acetylene. 21. Explain cis and trans addition with an exa ...
... 18. Explain the mechanism of markownikoff and antimarkownikoff addition of propene. 19. Write short notes on a)hydroboration reaction b) addition polymerization reaction 20. Explain the mode of hybridization of carbon in methane, ethylene and acetylene. 21. Explain cis and trans addition with an exa ...
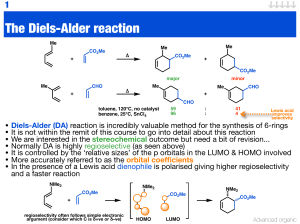
The Diels-Alder reaction
... the Cope rearrangement To minimise 1,3-diaxial interactions phenyl group is pseudo-equatorial Note: the original stereocentre is destroyed as the new centre is formed This process is often called ‘chirality transfer’ ...
... the Cope rearrangement To minimise 1,3-diaxial interactions phenyl group is pseudo-equatorial Note: the original stereocentre is destroyed as the new centre is formed This process is often called ‘chirality transfer’ ...
Stereoselective Construction of a β
... of t-BuLi used resulted in low conversion (entry 4). Interestingly, when the reaction of (E)-8 using 6.6 equiv of t-BuLi in the case of entry 3 was quenched with deuterium oxide, deuteriolysis occurred at the isopropenyl methyl moiety to provide the deuterated anti-9 in 80% d-content, indicating tha ...
... of t-BuLi used resulted in low conversion (entry 4). Interestingly, when the reaction of (E)-8 using 6.6 equiv of t-BuLi in the case of entry 3 was quenched with deuterium oxide, deuteriolysis occurred at the isopropenyl methyl moiety to provide the deuterated anti-9 in 80% d-content, indicating tha ...
Rapid, Controlled Assembly of Polyenes for Studying Pericyclic
... David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstanding examples of pericyclic reaction cascades are found in the biosyntheses of the ...
... David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstanding examples of pericyclic reaction cascades are found in the biosyntheses of the ...
Microsoft Word - Final Exam Study Guide
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
... stability, elimination reactions, Zaitsev’s rule, E1 mechanism, E2 mechanism, antiperiplanar, comparing substitution and elimination mechanisms, synthesis of ethers, alcohols, and epoxides, dehydration of alcohols, carbocation rearrangements, reactions of alcohols/ethers/epoxides, multistep synthesi ...
Chemistry 322 Experiment #3 Data Sheet
... 5. In the first part of this experiment, you performed Lucas tests on several alcohols. a) Write a complete mechanism for the reaction occurring between the Lucas reagent and tbutanol. ...
... 5. In the first part of this experiment, you performed Lucas tests on several alcohols. a) Write a complete mechanism for the reaction occurring between the Lucas reagent and tbutanol. ...
4.6, 4.7 test - A
... Cumene, C6H5CH(CH3)2, is the major organic product obtained when benzene and propene react together in the presence of aluminium chloride and hydrogen chloride. (a) ...
... Cumene, C6H5CH(CH3)2, is the major organic product obtained when benzene and propene react together in the presence of aluminium chloride and hydrogen chloride. (a) ...
doc
... Elimination reactions of alkanes (and substituted alkanes) (“Beta elimination reactions”, or “1,2 elimination reactions”) Dehydrogenation (at high T, 750C) Enthalpy is bad; entropy drives this ...
... Elimination reactions of alkanes (and substituted alkanes) (“Beta elimination reactions”, or “1,2 elimination reactions”) Dehydrogenation (at high T, 750C) Enthalpy is bad; entropy drives this ...
Final Exam Review Sheet Chemistry 110a/1998
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
... cation, and anion using a resonance and molecular orbital argument. How does the allylic radical compare in stability to 3°, 2°, and 1°? How about the allylic cation, in this regard? The pKa of an allylic hydrogen is 41: how can you use this value to say that the allylic anion is more stable than th ...
Exam 2
... -Be able to depict the reaction coordinate diagrams of each reaction -Be able to draw the mechanism, products and the stereochemical results of the Sn2, Sn1, E2 and E1 mechanism -If given a reaction be able to predict if it will favor the Sn2, Sn1, E2 or the E1 mechanism -Know how the incorporation ...
... -Be able to depict the reaction coordinate diagrams of each reaction -Be able to draw the mechanism, products and the stereochemical results of the Sn2, Sn1, E2 and E1 mechanism -If given a reaction be able to predict if it will favor the Sn2, Sn1, E2 or the E1 mechanism -Know how the incorporation ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.