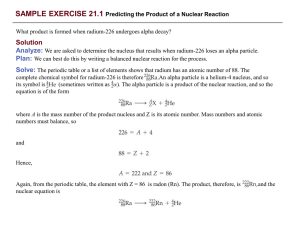
Notes - Science 2015-2016
... • Friday: Unit 1 Test • Monday: Unit 2: Intro to the Periodic Table ▫ This includes videos, coloring, etc. ...
... • Friday: Unit 1 Test • Monday: Unit 2: Intro to the Periodic Table ▫ This includes videos, coloring, etc. ...
atomic number - Net Start Class
... atomic mass, that is the weighted average mass of all an element’s isotopes. • Carbon-12 was used as a reference to measure mass of atoms, and the carbon-12 atom was said to have a mass of exactly 12 amu’s. ...
... atomic mass, that is the weighted average mass of all an element’s isotopes. • Carbon-12 was used as a reference to measure mass of atoms, and the carbon-12 atom was said to have a mass of exactly 12 amu’s. ...
SECTION 3-2: THE STRUCTURE OF THE ATOM
... 1. Nucleus: * Has at least one positively charged particle called a proton and generally one or more neutral particles called neutrons. * Very small region located near the center of the atom 2. Electrons: Surrounds the nucleus (electron cloud) and are negatively charged Protons, neutrons, and elect ...
... 1. Nucleus: * Has at least one positively charged particle called a proton and generally one or more neutral particles called neutrons. * Very small region located near the center of the atom 2. Electrons: Surrounds the nucleus (electron cloud) and are negatively charged Protons, neutrons, and elect ...
Document
... very stable. The especially stable. The nucleus does not have a magic number of either protons or neutrons. In fact, it has an odd number of both protons (43) and neutrons (55). There are very few stable nuclei with odd numbers of both protons and neutrons. Indeed, technetium-98 is radioactive. ...
... very stable. The especially stable. The nucleus does not have a magic number of either protons or neutrons. In fact, it has an odd number of both protons (43) and neutrons (55). There are very few stable nuclei with odd numbers of both protons and neutrons. Indeed, technetium-98 is radioactive. ...
Atomic Structure and Types of Atoms
... is the sum of the protons and neutrons in the nucleus of an atom. The most common isotope of carbon has a mass number of 12 (6 protons + 6 neutrons) and may be written as “carbon-12.” Two other isotopes are carbon-13 and carbon-14. As shown in Figure 3, a symbol with the mass number above and the at ...
... is the sum of the protons and neutrons in the nucleus of an atom. The most common isotope of carbon has a mass number of 12 (6 protons + 6 neutrons) and may be written as “carbon-12.” Two other isotopes are carbon-13 and carbon-14. As shown in Figure 3, a symbol with the mass number above and the at ...
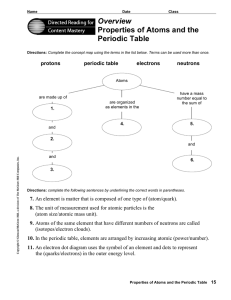
Reading Assignment Worksheet on Atoms - District 196 e
... 2. In this table, where are the metals located? 3. Where are the nonmetals located? 4. What are the elements in Groups 3 through 12 called? 5. What are the elements called that are next to the stairstep-shaped line on the right side of the table? 6. What do we call the letter or letters that represe ...
... 2. In this table, where are the metals located? 3. Where are the nonmetals located? 4. What are the elements in Groups 3 through 12 called? 5. What are the elements called that are next to the stairstep-shaped line on the right side of the table? 6. What do we call the letter or letters that represe ...
Journey from Bottle to Bang Insignificant though this bottle of
... 4 gentle little eruptions of particle tracks. Hydrogen atoms from this gas cylinder are fed at a precisely controlled rate into the source chamber of a linear accelerator – CERN’s Linac 2 – where their electrons are stripped off WIND RUSH to leave hydrogen nuclei. These are protons and have a positi ...
... 4 gentle little eruptions of particle tracks. Hydrogen atoms from this gas cylinder are fed at a precisely controlled rate into the source chamber of a linear accelerator – CERN’s Linac 2 – where their electrons are stripped off WIND RUSH to leave hydrogen nuclei. These are protons and have a positi ...
lecture notes - University of Chicago
... balance of neutrons to protons, the situation can often be corrected through the intervention of the ‘weak force’. The weak force is harder to picture than the other three fundamental forces, since it does not involve attraction or repulsion. It has such a short range that it essentially just op ...
... balance of neutrons to protons, the situation can often be corrected through the intervention of the ‘weak force’. The weak force is harder to picture than the other three fundamental forces, since it does not involve attraction or repulsion. It has such a short range that it essentially just op ...
PowerPoint - Models of the Atom
... 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus 3. The number of Protons = Number of Electrons. 4. Electrons orbit the nucleus in shells. 5. Each shell can only carry a set number of electrons. ...
... 1. The Atomic Number of an atom = number of protons in the nucleus. 2. The Atomic Mass of an atom = number of Protons + Neutrons in the nucleus 3. The number of Protons = Number of Electrons. 4. Electrons orbit the nucleus in shells. 5. Each shell can only carry a set number of electrons. ...
Chapter 10
... with a half-life of 12 hours. How much will remain in the body after 2.0 days, assuming that radioactive decay is the only path for removal of the isotope form the body. ...
... with a half-life of 12 hours. How much will remain in the body after 2.0 days, assuming that radioactive decay is the only path for removal of the isotope form the body. ...
Atom - Images
... (getting smaller) because the positive charge is greater than the smaller negative charges and pulls them in toward the nucleus. • More electrons than protons = radii increases (getting larger) because the electrons are farther away from the positive nucleus. • The Natural state of atoms has protons ...
... (getting smaller) because the positive charge is greater than the smaller negative charges and pulls them in toward the nucleus. • More electrons than protons = radii increases (getting larger) because the electrons are farther away from the positive nucleus. • The Natural state of atoms has protons ...
Pre-AP Review Unit 2
... 15. All atoms are neutral because the number of __________________ always equals the number of __________________ in every atom. Fill in the chart with a charge and mass: ...
... 15. All atoms are neutral because the number of __________________ always equals the number of __________________ in every atom. Fill in the chart with a charge and mass: ...
Physical Science Goal 5
... the # protons, # electrons, and # of neutrons: 1. Determine the Atomic # and Mass # 2. Atomic # = # protons a) # protons = # electrons ...
... the # protons, # electrons, and # of neutrons: 1. Determine the Atomic # and Mass # 2. Atomic # = # protons a) # protons = # electrons ...
different types of atoms
... Chemistry: Atomic Structure The three subatomic particles are: Proton – Positive charge Electron – Negative charge Neutron – No charge or neutral ...
... Chemistry: Atomic Structure The three subatomic particles are: Proton – Positive charge Electron – Negative charge Neutron – No charge or neutral ...
and View
... b. Isotopes—atoms of same element that have different numbers of neutrons. Ex: carbon-12, carbon-13, carbon-14 c. Mass number—number of neutrons plus protons in an atom. i. Neutron number is found by--Mass number - Atomic number _______________ Number of neutrons ...
... b. Isotopes—atoms of same element that have different numbers of neutrons. Ex: carbon-12, carbon-13, carbon-14 c. Mass number—number of neutrons plus protons in an atom. i. Neutron number is found by--Mass number - Atomic number _______________ Number of neutrons ...
Unit 3 Rev Pckt - Old Saybrook Public Schools
... b. positive charges are dispersed throughout the atom. c. positive charges are concentrated in a very small core at the atom's center. d. protons and neutrons are located in the nucleus. 23. Scientists have determined that electrons a. move in space around the nucleus. b. have a mass equal to the ma ...
... b. positive charges are dispersed throughout the atom. c. positive charges are concentrated in a very small core at the atom's center. d. protons and neutrons are located in the nucleus. 23. Scientists have determined that electrons a. move in space around the nucleus. b. have a mass equal to the ma ...
21J 2011 The Polywell Nuclear Reactor Website July 4, 2011
... The mass numbers given in the table are the known stable isotopes, which do not break down spontaneously. Unstable isotopes are not shown because there are too many of them. When unstable isotopes break down into new isotopes, they usually emit alpha, beta, or gamma radiation. It says “none” above U ...
... The mass numbers given in the table are the known stable isotopes, which do not break down spontaneously. Unstable isotopes are not shown because there are too many of them. When unstable isotopes break down into new isotopes, they usually emit alpha, beta, or gamma radiation. It says “none” above U ...
Atomic structure
... Elements are made up of one type of atom, but there can be slightly different forms of the atoms in an element. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that mass number is different called isotopes For example, tw ...
... Elements are made up of one type of atom, but there can be slightly different forms of the atoms in an element. Although atoms of the same element always have the same number of protons, they may have different numbers of neutrons. Atoms that mass number is different called isotopes For example, tw ...
atom - Ector County ISD
... blast of thermo-nuclear bombs. Nuclear energy results from changes in the nucleus of atoms. The first atomic bomb, or A-bomb, exploded on July 16, 1945 in Alamogordo, New Mexico. It produced and explosion equal to that of 19,000 short tons (17,000 metric tons) of TNT. ...
... blast of thermo-nuclear bombs. Nuclear energy results from changes in the nucleus of atoms. The first atomic bomb, or A-bomb, exploded on July 16, 1945 in Alamogordo, New Mexico. It produced and explosion equal to that of 19,000 short tons (17,000 metric tons) of TNT. ...
Chapter 4 - Germainium.net
... mass of 78.918336 amu and occupying 50.69% and the second isotope having a mass of 80.916289 amu and occupying 49.31%. What is the average atomic mass of bromine? • Verify the atomic mass of Magnesium: 24Mg = 23.985042 amu and percent abundance of 78.99% , 25Mg = 24.985837 amu and percent abundance ...
... mass of 78.918336 amu and occupying 50.69% and the second isotope having a mass of 80.916289 amu and occupying 49.31%. What is the average atomic mass of bromine? • Verify the atomic mass of Magnesium: 24Mg = 23.985042 amu and percent abundance of 78.99% , 25Mg = 24.985837 amu and percent abundance ...
Honors Chem: Atomic History-Isotopes
... were used to make 12 grams of compound Z, how many grams of element X were required? According to the law of conservation of mass, if element A has a mass of 2 mass units, and element B has a mass of 3 mass units, what mass would be expected for compound AB? State the law of multiple proportions [co ...
... were used to make 12 grams of compound Z, how many grams of element X were required? According to the law of conservation of mass, if element A has a mass of 2 mass units, and element B has a mass of 3 mass units, what mass would be expected for compound AB? State the law of multiple proportions [co ...
Make a large atom with p:95, n:146, e:95 - TSDCurriculum
... scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. The prefix is "iso" means "same" and they should have used "prot" for protons. The new word woul ...
... scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. The prefix is "iso" means "same" and they should have used "prot" for protons. The new word woul ...
Radioactivity
... You can think of different isotopes of an atom being different "versions" of that atom. • Consider a carbon atom. It has 6 protons and 6 neutrons - we call it "carbon-12" because it has an atomic mass of 12 (6 plus 6). If we add a neutron, it's still a carbon atom, but it's a different isotope of ca ...
... You can think of different isotopes of an atom being different "versions" of that atom. • Consider a carbon atom. It has 6 protons and 6 neutrons - we call it "carbon-12" because it has an atomic mass of 12 (6 plus 6). If we add a neutron, it's still a carbon atom, but it's a different isotope of ca ...