Atomic Structure and the Periodic Table
... _______________, they too are made up of even smaller particles. Atoms are made up of 3 _____________ particles: ___________, _____________, and _______________. ...
... _______________, they too are made up of even smaller particles. Atoms are made up of 3 _____________ particles: ___________, _____________, and _______________. ...
Counting Atoms - Effingham County Schools
... How many protons, electrons, and neutrons are there in an atom of chlorine-37? Given: name and mass number of chlorine-37 Remember: atomic number = number of protons = number of electrons From the periodic table, the atomic number of chlorine is 17. Mass # - atomic # = # of neutrons 37 – 17 = 20 An ...
... How many protons, electrons, and neutrons are there in an atom of chlorine-37? Given: name and mass number of chlorine-37 Remember: atomic number = number of protons = number of electrons From the periodic table, the atomic number of chlorine is 17. Mass # - atomic # = # of neutrons 37 – 17 = 20 An ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
Atomic Structure Study Guide
... Gold Foil Experiment: Marsden aimed a narrow beam of alpha particles at the gold. The screen around the gold was made of a material that produced a flash of light when struck by a fast-moving alpha particle. By observing the flash, Marsden could figure out the path of an alpha particle after it pass ...
... Gold Foil Experiment: Marsden aimed a narrow beam of alpha particles at the gold. The screen around the gold was made of a material that produced a flash of light when struck by a fast-moving alpha particle. By observing the flash, Marsden could figure out the path of an alpha particle after it pass ...
Atoms - Issaquah Connect
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
... • ALL atoms of the same element have the same number of protons. • All neutral atoms have no overall (net) charge, so … have the same number of electrons as protons • BUT… they can have different numbers of neutrons These are called isotopes of carbon ...
A2_Unit5_Nuclear_13_Binding_Energy
... Iron has the highest binding energy per nucleon so is the most stable nucleus. If we look at large nuclei (greater than iron), we find that the further to the right (greater nucleon number) the less stable the nuclei. This is because the binding energy per nucleon is getting less. The explanation fo ...
... Iron has the highest binding energy per nucleon so is the most stable nucleus. If we look at large nuclei (greater than iron), we find that the further to the right (greater nucleon number) the less stable the nuclei. This is because the binding energy per nucleon is getting less. The explanation fo ...
Unit 3 - Section 5.1 Introduction to Chemistry
... deuterium (1 neutron) and tritium (2 neutrons). Since neutrons has no electrical charge, the chemistry of the element is not impacted. However, the mass of the element changes. Isotopes can be stable or unstable. Unstable isotopes decay. They are radioactive. NOTE: All anthropogenic elements are rad ...
... deuterium (1 neutron) and tritium (2 neutrons). Since neutrons has no electrical charge, the chemistry of the element is not impacted. However, the mass of the element changes. Isotopes can be stable or unstable. Unstable isotopes decay. They are radioactive. NOTE: All anthropogenic elements are rad ...
Chapter 5
... In our class, we are always going to round average atomic masses to 1 decimal place. So, we’ll round 35.49 to 35.5 and that’s the average atomic mass of Chlorine that we’ll use. Why can’t you just average 35 and 37 (the two isotopes) and get 36 as the average atomic mass? Why is that wrong? ...
... In our class, we are always going to round average atomic masses to 1 decimal place. So, we’ll round 35.49 to 35.5 and that’s the average atomic mass of Chlorine that we’ll use. Why can’t you just average 35 and 37 (the two isotopes) and get 36 as the average atomic mass? Why is that wrong? ...
atoms
... • atomic number = number of protons • in a neutral atom, the # of protons = the # of electrons • atomic mass= the number of protons + the number of ...
... • atomic number = number of protons • in a neutral atom, the # of protons = the # of electrons • atomic mass= the number of protons + the number of ...
File - GarzScience!
... • Experimental evidence led to general acceptance of his atomic theory! • Not all of the theory was accurate. • It has had to be revised with new information ...
... • Experimental evidence led to general acceptance of his atomic theory! • Not all of the theory was accurate. • It has had to be revised with new information ...
Atom - WCHS Physical Science
... Modern Model of atom (wave model) • Based on probability • Says that an electron’s location can’t be determined exactly ...
... Modern Model of atom (wave model) • Based on probability • Says that an electron’s location can’t be determined exactly ...
Histroy_of_the_Atom
... The nucleus is the tiny positive core of the atom which contains most of the mass of the atom. The proton (p+) is the positively (1+) charged particle found in the nucleus of the atom. The neutron (no) is the particle with no charge (0) found in the nucleus of the atom. ...
... The nucleus is the tiny positive core of the atom which contains most of the mass of the atom. The proton (p+) is the positively (1+) charged particle found in the nucleus of the atom. The neutron (no) is the particle with no charge (0) found in the nucleus of the atom. ...
atomic mass.
... If there were only lots of positive charges (protons) in the nucleus very tightly packed wouldn’t they repel each other like magnets of the same charge. There must be something in the nucleus, but in between the protons to space them out. ...
... If there were only lots of positive charges (protons) in the nucleus very tightly packed wouldn’t they repel each other like magnets of the same charge. There must be something in the nucleus, but in between the protons to space them out. ...
Unit 3 GROUP QUIZ
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
... b. Electrons are negatively charged and have a mass of 1 u. c. The nucleus of an atom is positively charged. d. The neutron is found in the nucleus of an atom. ___11. All atoms are ______. a. positively charged, with the number of protons exceeding the number of electrons. b. Negatively charged, wit ...
File
... Law of Conservation of Mass: mass is neither created or destroyed in ordinary chemical reactions Law of Definite Proportions (composition): compounds contain same elements in same ratio by mass Example: NaCl is always 39.9% Na and 60.66% Cl by mass Law of Multiple Proportions: 2 or more different co ...
... Law of Conservation of Mass: mass is neither created or destroyed in ordinary chemical reactions Law of Definite Proportions (composition): compounds contain same elements in same ratio by mass Example: NaCl is always 39.9% Na and 60.66% Cl by mass Law of Multiple Proportions: 2 or more different co ...
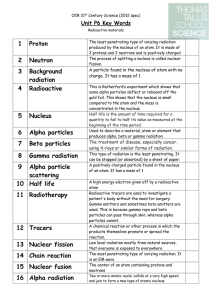
3 Background radiation
... A high energy electron given off by a radioactive atom Radioactive tracers are used to investigate a patient's body without the need for surgery. Gamma emitters and sometimes beta emitters are used. This is because gamma rays and beta particles can pass through skin, whereas alpha particles cannot. ...
... A high energy electron given off by a radioactive atom Radioactive tracers are used to investigate a patient's body without the need for surgery. Gamma emitters and sometimes beta emitters are used. This is because gamma rays and beta particles can pass through skin, whereas alpha particles cannot. ...
WAHS—Chemistry Unit 4: Atomic Structure 1 Unit Assignment #1
... 12. How do the three isotopes of hydrogen (H–1, H–2, H–3) compare in terms of the numbers of subatomic particles in each? 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. ...
... 12. How do the three isotopes of hydrogen (H–1, H–2, H–3) compare in terms of the numbers of subatomic particles in each? 13. Write the nuclear symbol for deuterium (H-2): a. Identify the atomic number b. Identify the mass number 14. Determine the number of protons, neutrons, and electrons in Co–59. ...
Atomic Structure and Isotopes
... Each element in the periodic table has a unique atomic number. The atomic number corresponds to the number of protons in the nucleus. For an element, the number of electrons will equal the number of protons. ...
... Each element in the periodic table has a unique atomic number. The atomic number corresponds to the number of protons in the nucleus. For an element, the number of electrons will equal the number of protons. ...
The Mighty Electron - MVUSD Haiku Learning
... minus sign, its an ion! If there is no sign, its neutral. • P = neutral atom of phosphorous • O-2 = ion of oxygen w/a -2 charge • Li+ = ion of lithium w/a +1 charge ...
... minus sign, its an ion! If there is no sign, its neutral. • P = neutral atom of phosphorous • O-2 = ion of oxygen w/a -2 charge • Li+ = ion of lithium w/a +1 charge ...
Atomic Origins: Chapter Problems Big Bang Class Work How old is
... made up mostly of empty space. The electrons orbited the nucleus. The atoms were heavier than expected leading to the discovery of neutrons inside the nucleus. 2. Unknown element a. Titanium because the majority of this element’s atoms have an atomic mass of 47.9480 u. The element on the periodic ta ...
... made up mostly of empty space. The electrons orbited the nucleus. The atoms were heavier than expected leading to the discovery of neutrons inside the nucleus. 2. Unknown element a. Titanium because the majority of this element’s atoms have an atomic mass of 47.9480 u. The element on the periodic ta ...
atoms
... The first ring can hold = 2 electrons. The second ring can hold = 8 electrons The third ring can hold = 18 electrons ...
... The first ring can hold = 2 electrons. The second ring can hold = 8 electrons The third ring can hold = 18 electrons ...