ap chemistry 2005/2006
... A typical week is organized to provide: 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections ...
... A typical week is organized to provide: 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections ...
Indian Journal of Chemistry
... signals in some classes of polynuclear aromatic hydrocarbons, viz., linear polyacenes, flanked pyrenes, triangulenes and rhombulenes have been deduced using Pólya’s enumeration theorem (PET) and Balasubramanian’s method of reduced cycle indices. Given only a very obvious structural feature of the mo ...
... signals in some classes of polynuclear aromatic hydrocarbons, viz., linear polyacenes, flanked pyrenes, triangulenes and rhombulenes have been deduced using Pólya’s enumeration theorem (PET) and Balasubramanian’s method of reduced cycle indices. Given only a very obvious structural feature of the mo ...
Chem expo 12
... Industrial chemistry, which focuses on the factors that affect the rate and extent of chemical reactions. Students study energy profiles and how equilibrium laws are applied to homogeneous equilibria. Experiments are conducted to investigate the effect of temperature, concentration of reagents, pres ...
... Industrial chemistry, which focuses on the factors that affect the rate and extent of chemical reactions. Students study energy profiles and how equilibrium laws are applied to homogeneous equilibria. Experiments are conducted to investigate the effect of temperature, concentration of reagents, pres ...
Chemistry 2014 - SC3210 IC Scope and Sequence
... Science Practice: Classify matter as pure substances or mixtures by studying their properties. Mixtures and Solutions Describe heterogeneous mixtures, including suspensions and colloids. Describe homogeneous mixtures, such as solutions. Identify nonaqueous solutions. Identify the components of a sol ...
... Science Practice: Classify matter as pure substances or mixtures by studying their properties. Mixtures and Solutions Describe heterogeneous mixtures, including suspensions and colloids. Describe homogeneous mixtures, such as solutions. Identify nonaqueous solutions. Identify the components of a sol ...
Briefing Session on 2012 HKDSE Examination (December 2012)
... A technician wore a pair of gloves ... natural rubber and performed an experiment involving bromine. ... brittle ... Explain the phenomenon. (2 marks, fairly answered) ✔ Addition of bromine to the C=C bonds of the materials ✔ The brominated polymer have weaker intramolecular structure / weaker inter ...
... A technician wore a pair of gloves ... natural rubber and performed an experiment involving bromine. ... brittle ... Explain the phenomenon. (2 marks, fairly answered) ✔ Addition of bromine to the C=C bonds of the materials ✔ The brominated polymer have weaker intramolecular structure / weaker inter ...
Section 4.8: Acid-Base Reactions
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions in a precipitation reaction will keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions in a precipitation reaction will keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
Unit 6: Reactions and Stoichiometry
... At the most fundamental level, the chemist needs a unit that describes a very large quantity. One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined ...
... At the most fundamental level, the chemist needs a unit that describes a very large quantity. One of the most well-known numbers in the study of chemistry is number of units in a mole. The number of units in a mole is called Avogadro’s number (named after the Italian physicist). The mole is defined ...
Examination - SCSA - School Curriculum and Standards Authority
... DO NOT WRITE IN THIS AREA AS IT WILL BE CUT OFF See next page ...
... DO NOT WRITE IN THIS AREA AS IT WILL BE CUT OFF See next page ...
Practice Problems in Biomedical Organic Chemistry
... to serve as self-directed learning tools, which students can use at a pace that meets their individual educational needs. We have created three types of problems (i.e., C, I, A) for each topic: C: Core concepts. These problems define major terms and ideas pertaining to a particular topic. I: Int ...
... to serve as self-directed learning tools, which students can use at a pace that meets their individual educational needs. We have created three types of problems (i.e., C, I, A) for each topic: C: Core concepts. These problems define major terms and ideas pertaining to a particular topic. I: Int ...
Course Description Word File
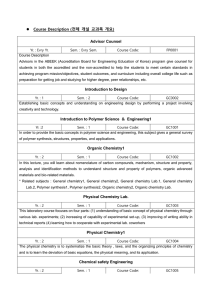
... Instrumental Polymer Analysis2 Yr. : 3 Sem. : 2 Course Code: GC1020 To educate understanding and analysis ability of characteristics of polymer materials by understanding fundamental principles and concepts of characterizing various thermal, mechanical, microscopic, rheological properties of polymer ...
... Instrumental Polymer Analysis2 Yr. : 3 Sem. : 2 Course Code: GC1020 To educate understanding and analysis ability of characteristics of polymer materials by understanding fundamental principles and concepts of characterizing various thermal, mechanical, microscopic, rheological properties of polymer ...
Brochure BITSAT-2011
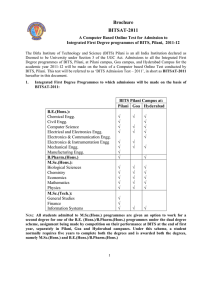
... All the questions and instructions of the test will be in English only. Candidates should bring a pen for the purpose of rough work, signing etc. Blank sheets for rough work will be provided, if required. Calculators and logarithmic tables are not allowed in the test centers. Candidates are not allo ...
... All the questions and instructions of the test will be in English only. Candidates should bring a pen for the purpose of rough work, signing etc. Blank sheets for rough work will be provided, if required. Calculators and logarithmic tables are not allowed in the test centers. Candidates are not allo ...
Itty-Bitty Atoms
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
... science? Would you like to be a scientist? If so, what would you like to study? What do you think scientists of the future will study? 4. Answer the following questions: a. Who is Dmitry Mendeleyev and what did he do? b. What is chemistry? c. How big are atoms? 5. When a teacher calls out a symbol f ...
3. d-Block elements. Biological role, application in medicine.
... Mg, Ca, Sr, Ba, Ra as well as hydrogen and helium belong to the block of selements. The electronic formula of the external shell of IA-group elements and hydrogen is ns1 and of the elements of IIA group and helium - ns2, where “n” is the number of the period. Chemical properties of s-elements of IA ...
... Mg, Ca, Sr, Ba, Ra as well as hydrogen and helium belong to the block of selements. The electronic formula of the external shell of IA-group elements and hydrogen is ns1 and of the elements of IIA group and helium - ns2, where “n” is the number of the period. Chemical properties of s-elements of IA ...
biogenic s, p, d-block elements, biological role, application in medicine
... Mg, Ca, Sr, Ba, Ra as well as hydrogen and helium belong to the block of selements. The electronic formula of the external shell of IA-group elements and hydrogen is ns1 and of the elements of IIA group and helium - ns2, where “n” is the number of the period. Chemical properties of s-elements of IA ...
... Mg, Ca, Sr, Ba, Ra as well as hydrogen and helium belong to the block of selements. The electronic formula of the external shell of IA-group elements and hydrogen is ns1 and of the elements of IIA group and helium - ns2, where “n” is the number of the period. Chemical properties of s-elements of IA ...
ap chemistry 2005/2006
... A typical week is organized to provide: 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections ...
... A typical week is organized to provide: 3-4 days of lecture focused on the key objectives listed in the syllabus, including teacher demonstrations 1-2 days of lab activity. Labs may exceed one 90 minute class, depending on the requirements of the specific lab activity. In addition, some sections ...
Chapter 1: Biochemistry in the Modern World
... became interested in studying the chemical reactions that occur in living organisms. These chemists discovered, back in the nineteenth century, that biochemistry presents its own unique challenges. Foremost among these is the complexity of the mixture of molecules that are present in a living cell. ...
... became interested in studying the chemical reactions that occur in living organisms. These chemists discovered, back in the nineteenth century, that biochemistry presents its own unique challenges. Foremost among these is the complexity of the mixture of molecules that are present in a living cell. ...
Japanese Agricultural Standard for Organic Plants (Notification No
... plants); or an appropriate combination of these methods. In case of imminent or serious threat to plants and where cultural physical, biological controls, or any appropriate combination of them are not effective, substances for plant pest and disease control listed in Attached Table 2 (except for th ...
... plants); or an appropriate combination of these methods. In case of imminent or serious threat to plants and where cultural physical, biological controls, or any appropriate combination of them are not effective, substances for plant pest and disease control listed in Attached Table 2 (except for th ...
Chapter 3 Chemical Compounds
... (b) The ions present are the chromium(III) ion (Cr3+) and the nitride ion (the ‘-ide’ ending tells you this is a monatomic anion of nitrogen; since N is in column 5A, its negative charge is 8 – 5 = 3 or N3–), so this is chromium(III) nitride. Since chromium can have multiple charges (+2 or +3), the ...
... (b) The ions present are the chromium(III) ion (Cr3+) and the nitride ion (the ‘-ide’ ending tells you this is a monatomic anion of nitrogen; since N is in column 5A, its negative charge is 8 – 5 = 3 or N3–), so this is chromium(III) nitride. Since chromium can have multiple charges (+2 or +3), the ...
Chemistry - Pearson School
... This document demonstrates how Pearson Chemistry ©2012 meets the objectives of the New York Physical Setting/Chemistry Core Curriculum. Correlation page references are to the Student and Teacher’s Editions and are cited at the page level. Pearson Chemistry combines proven and tested content with cut ...
... This document demonstrates how Pearson Chemistry ©2012 meets the objectives of the New York Physical Setting/Chemistry Core Curriculum. Correlation page references are to the Student and Teacher’s Editions and are cited at the page level. Pearson Chemistry combines proven and tested content with cut ...
the Language of Chemistry
... Some questions that I have been often asked by the students and parents alike have been—“What do we do by learning Chemistry?” “For a student of Literature or even History what role can Chemistry play?” But the answers came very simply. A mother administering her child a tablet for fever should know ...
... Some questions that I have been often asked by the students and parents alike have been—“What do we do by learning Chemistry?” “For a student of Literature or even History what role can Chemistry play?” But the answers came very simply. A mother administering her child a tablet for fever should know ...
Can Naturalistic Evolution Explain the Origin of Life on Earth
... obviously essential for RNA, and hence for the RNA-world hypothesis of the origin of life.10 A team including the famous evolutionary origin-of-life pioneer Stanley Miller, in PNAS, found that the half life (t1/2) of ribose is only 44 years at pH 7.0 (neutral) and 0°C. It’s even worse at high temper ...
... obviously essential for RNA, and hence for the RNA-world hypothesis of the origin of life.10 A team including the famous evolutionary origin-of-life pioneer Stanley Miller, in PNAS, found that the half life (t1/2) of ribose is only 44 years at pH 7.0 (neutral) and 0°C. It’s even worse at high temper ...
B.Sc. (Hons.) Chemistry
... Strong, moderate and weak electrolytes, degree of ionization, factors affecting degree of ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect; dissociation constants of mono-, di-and triprotic acids (exact treatment). Salt hydro ...
... Strong, moderate and weak electrolytes, degree of ionization, factors affecting degree of ionization, ionization constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect; dissociation constants of mono-, di-and triprotic acids (exact treatment). Salt hydro ...
Questions
... A useful test for carboxylic acids is that they will neutralise sodium carbonate solution. Write a balanced equation, including state symbols, for the neutralisation of sodium carbonate solution by propanoic acid. ...
... A useful test for carboxylic acids is that they will neutralise sodium carbonate solution. Write a balanced equation, including state symbols, for the neutralisation of sodium carbonate solution by propanoic acid. ...
The Atomic Molecular Theory
... These statements are summaries of many observations, which required a tremendous amount of experimentation to achieve and even more creative thinking to systematize as we have written them here. By making these assumptions, we can proceed directly with the experiments which led to the development of ...
... These statements are summaries of many observations, which required a tremendous amount of experimentation to achieve and even more creative thinking to systematize as we have written them here. By making these assumptions, we can proceed directly with the experiments which led to the development of ...
Organic chemistry

Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study.The range of chemicals studied in organic chemistry include hydrocarbons (compounds containing only carbon and hydrogen), as well as myriad compositions based always on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (these, included in many organic chemicals in biology) and the radiostable elements of the halogens.In the modern era, the range extends further into the periodic table, with main group elements, including:Group 1 and 2 organometallic compounds, i.e., involving alkali (e.g., lithium, sodium, and potassium) or alkaline earth metals (e.g., magnesium)Metalloids (e.g., boron and silicon) or other metals (e.g., aluminium and tin)In addition, much modern research focuses on organic chemistry involving further organometallics, including the lanthanides, but especially the transition metals; (e.g., zinc, copper, palladium, nickel, cobalt, titanium and chromium)Finally, organic compounds form the basis of all earthly life and constitute a significant part of human endeavors in chemistry. The bonding patterns open to carbon, with its valence of four—formal single, double, and triple bonds, as well as various structures with delocalized electrons—make the array of organic compounds structurally diverse, and their range of applications enormous. They either form the basis of, or are important constituents of, many commercial products including pharmaceuticals; petrochemicals and products made from them (including lubricants, solvents, etc.); plastics; fuels and explosives; etc. As indicated, the study of organic chemistry overlaps with organometallic chemistry and biochemistry, but also with medicinal chemistry, polymer chemistry, as well as many aspects of materials science.