Preview Sample 2
... You also notice that the electrons in H2 are evenly distributed among the two atoms. Which two types of bonds are represented in these molecules? A. Covalent bonds in NaCl; ionic bonds in H2. B. Covalent bonds in NaCl; covalent bonds in H2. C. Ionic bonds in NaCl; ionic bonds in H2. D. Ionic bonds i ...
... You also notice that the electrons in H2 are evenly distributed among the two atoms. Which two types of bonds are represented in these molecules? A. Covalent bonds in NaCl; ionic bonds in H2. B. Covalent bonds in NaCl; covalent bonds in H2. C. Ionic bonds in NaCl; ionic bonds in H2. D. Ionic bonds i ...
5. Coenzyme HAD+ is derived
... -determine the amount of adsorption, coagulation threshold, the degree of swelling of biopolymers, the relative molecular mass of low molecular weight substances by cryometric data and macromolecular compounds by viscometric data; -obtain colloidal solutions and investigate their properties; -classi ...
... -determine the amount of adsorption, coagulation threshold, the degree of swelling of biopolymers, the relative molecular mass of low molecular weight substances by cryometric data and macromolecular compounds by viscometric data; -obtain colloidal solutions and investigate their properties; -classi ...
An Efficient Oxidation of Benzoins to Benzils by Manganese (II
... Transition metal catalysts supported by Schiff base ligands have assumed a prominent role in modern synthesis. Schiff base complexes of transition metals having O and N donor atoms have shown an exponential increase as inorganic catalysts for various organic transformations. Distinct advantages of suc ...
... Transition metal catalysts supported by Schiff base ligands have assumed a prominent role in modern synthesis. Schiff base complexes of transition metals having O and N donor atoms have shown an exponential increase as inorganic catalysts for various organic transformations. Distinct advantages of suc ...
selected experiments in organic chemistry
... will therefore serve the needs of non-chemistry majors (pharmacy, biology, agriculture etc.) requiring an introductory course in experimental organic chemistry. Chemistry majors may also benefit from this manual if it is used as an introductory course to be followed by more advanced laboratory techn ...
... will therefore serve the needs of non-chemistry majors (pharmacy, biology, agriculture etc.) requiring an introductory course in experimental organic chemistry. Chemistry majors may also benefit from this manual if it is used as an introductory course to be followed by more advanced laboratory techn ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • Homogeneous mixtures of two or more pure substances. • The solvent is present in greatest abundance. • All other substances are solutes. ...
... • Homogeneous mixtures of two or more pure substances. • The solvent is present in greatest abundance. • All other substances are solutes. ...
Review Unit: Chemistry Review
... or dangerous consequences. In fact, the comfortable lives we lead are due in large part to our understanding and application of chemistry. Some chemicals are harmful to people or the environment, but many are integral to life, such as the carbon dioxide, oxygen, water, and glucose in the cycle of ph ...
... or dangerous consequences. In fact, the comfortable lives we lead are due in large part to our understanding and application of chemistry. Some chemicals are harmful to people or the environment, but many are integral to life, such as the carbon dioxide, oxygen, water, and glucose in the cycle of ph ...
contact - DTU Kemi
... will interfere with the job of the other, as the effect of the Brønsted acid catalysis will set in only after the ruthenium hydride catalytic process has created the relevant compound for the other catalyst to work on. “In other words, the synthesis can be performed as a one-pot process,” David Tann ...
... will interfere with the job of the other, as the effect of the Brønsted acid catalysis will set in only after the ruthenium hydride catalytic process has created the relevant compound for the other catalyst to work on. “In other words, the synthesis can be performed as a one-pot process,” David Tann ...
Name: Date: ______ 1. Which of the following is a property of both
... (1) A basis for distinguishing between an element and a compound is whether the substance can be decomposed into other substances using chemical means. (2) Current chemical theory strongly suggests that all naturally occurring elements have been identified. (3) The elements silver, gold, and aluminu ...
... (1) A basis for distinguishing between an element and a compound is whether the substance can be decomposed into other substances using chemical means. (2) Current chemical theory strongly suggests that all naturally occurring elements have been identified. (3) The elements silver, gold, and aluminu ...
Aromatic Chemistry - heckgrammar.co.uk
... the order of the reaction regarding each reagent can provide information regarding its involvement in the rate determining step obviously a reactant with 0 order will not be involved in the rate determining step study of reaction kinetics can yield important information regarding the mechanism of a ...
... the order of the reaction regarding each reagent can provide information regarding its involvement in the rate determining step obviously a reactant with 0 order will not be involved in the rate determining step study of reaction kinetics can yield important information regarding the mechanism of a ...
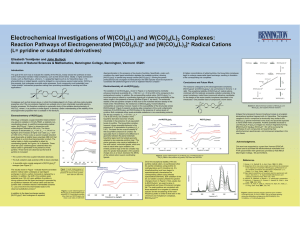
Electrochemical Investigations of W(CO) (L) and W(CO) (L) Complexes:
... irreversible in the presence of sub-equivalent levels of pyridine. The bis-pyridine complex, however, shows reversible behavior in the presence of 10 equivalents of pyridine (ic/ia = 0.87). The basis for the unusual stability of the bis-pyridine compound is unclear; we speculate that it is related t ...
... irreversible in the presence of sub-equivalent levels of pyridine. The bis-pyridine complex, however, shows reversible behavior in the presence of 10 equivalents of pyridine (ic/ia = 0.87). The basis for the unusual stability of the bis-pyridine compound is unclear; we speculate that it is related t ...
Prentice Hall Ch 02 Atoms Molecules Ions
... front cover to determine which of the three is the most abundant. Can you determine which is the second most abundant? Explain. ...
... front cover to determine which of the three is the most abundant. Can you determine which is the second most abundant? Explain. ...
The First Steps of Chemical Evolution towards the
... [33] has already proposed a similar scenario for the appearance of RNA and DNA in evolution, and the considerations presented here seem to support this hypothesis. We certainly have to expect that the old Gprotein worldF has vanished and been replaced by the modern life forms based on polynucleotide ...
... [33] has already proposed a similar scenario for the appearance of RNA and DNA in evolution, and the considerations presented here seem to support this hypothesis. We certainly have to expect that the old Gprotein worldF has vanished and been replaced by the modern life forms based on polynucleotide ...
BC Science 10 Workbook Answers
... bacteria, using a series of chemical reactions, convert nitrate back into nitrogen gas. 8. Eutrophication is the process by which excess nutrients result in increased plant production and decay in aquatic ecosystems. Interpreting Illustrations ...
... bacteria, using a series of chemical reactions, convert nitrate back into nitrogen gas. 8. Eutrophication is the process by which excess nutrients result in increased plant production and decay in aquatic ecosystems. Interpreting Illustrations ...
in MS Word - The Natural Edge Project
... Design chemical reactions so that as many of the atoms as possible that are present in the starting materials end up in the product rather than in the waste stream – i.e. being atom efficient, and thus preventing waste production.18 ...
... Design chemical reactions so that as many of the atoms as possible that are present in the starting materials end up in the product rather than in the waste stream – i.e. being atom efficient, and thus preventing waste production.18 ...
`A` LEVEL H2 CHEMISTRY ORGANIC REACTIONS SUMMARY By
... The graph below shows how pV changes with p for the three gases, J, K and L, at a fixed temperature (where p = pressure, V = volume). ...
... The graph below shows how pV changes with p for the three gases, J, K and L, at a fixed temperature (where p = pressure, V = volume). ...
Development of Novel Catalytic Asymmetric Reactions using
... (La, Zn, Ca, R4N+) directly from ketones. The latter approach features high atom efficiency, and as with organocatalysis, dramatic advances have been made in this area in recent years.2 ...
... (La, Zn, Ca, R4N+) directly from ketones. The latter approach features high atom efficiency, and as with organocatalysis, dramatic advances have been made in this area in recent years.2 ...
Radiation Chemistry of Overirradiated Aqueous Solutions of
... Gas chromatography shows the presence of dicarboxylic and tricarboxylic acids (Fig. 6). They were found also at absorbed doses lower than 11 Mrad in our earlier work (Negr6n-Mendoza et al. 1983); the report of that work also gives more details about their identification and considers possible pathwa ...
... Gas chromatography shows the presence of dicarboxylic and tricarboxylic acids (Fig. 6). They were found also at absorbed doses lower than 11 Mrad in our earlier work (Negr6n-Mendoza et al. 1983); the report of that work also gives more details about their identification and considers possible pathwa ...
Learning Outcomes
... of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ......................................................................................................................... 16 (c) define proton (atomic) number and nucleon (mass) number ...
... of s, p, d and f classification is not required; a copy of the Periodic Table will be available in Papers 1 and 2) ......................................................................................................................... 16 (c) define proton (atomic) number and nucleon (mass) number ...
department of pure and applied chemistry
... service of God and humanity. Chemistry is the science that deals with the nature of matter. Chemistry is fundamental to all other sciences; it is the centre science which leads to the understanding of matter, hence chemistry is studied as pure science as well as an applied science; in the first case ...
... service of God and humanity. Chemistry is the science that deals with the nature of matter. Chemistry is fundamental to all other sciences; it is the centre science which leads to the understanding of matter, hence chemistry is studied as pure science as well as an applied science; in the first case ...
Nordonia Hills City Schools Honors Chemistry Course of Study
... Determine the number of protons, electrons and neutrons; write nuclide symbols Describe properties, names, and location of subatomic particles. Compare and contrast contributors to early atomic theory: Greeks, Dalton, Thomson, Rutherford, Chadwick, and Bohr) Describe concepts involved in Dalton's po ...
... Determine the number of protons, electrons and neutrons; write nuclide symbols Describe properties, names, and location of subatomic particles. Compare and contrast contributors to early atomic theory: Greeks, Dalton, Thomson, Rutherford, Chadwick, and Bohr) Describe concepts involved in Dalton's po ...
Chemical Reaction
... breaks down to form two or more simpler substances. (synonyms: corrode, decay, breakdown) • Single Displacement: Sometimes, an element replaces another element that is a part of a compound. This type of reaction is called a single-displacement reaction. (synonyms: move, shift, rearrange) • Double Di ...
... breaks down to form two or more simpler substances. (synonyms: corrode, decay, breakdown) • Single Displacement: Sometimes, an element replaces another element that is a part of a compound. This type of reaction is called a single-displacement reaction. (synonyms: move, shift, rearrange) • Double Di ...
Chemistry
... This course deals with the major topics of concern in environmental chemistry. Emphasis is placed on the chemistry involved, as well as assessment of the relative hazards and corrective methods available to provide abatement. Topics covered include: atmospheric free radical chemistry, the green- hou ...
... This course deals with the major topics of concern in environmental chemistry. Emphasis is placed on the chemistry involved, as well as assessment of the relative hazards and corrective methods available to provide abatement. Topics covered include: atmospheric free radical chemistry, the green- hou ...
Organic chemistry

Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study.The range of chemicals studied in organic chemistry include hydrocarbons (compounds containing only carbon and hydrogen), as well as myriad compositions based always on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (these, included in many organic chemicals in biology) and the radiostable elements of the halogens.In the modern era, the range extends further into the periodic table, with main group elements, including:Group 1 and 2 organometallic compounds, i.e., involving alkali (e.g., lithium, sodium, and potassium) or alkaline earth metals (e.g., magnesium)Metalloids (e.g., boron and silicon) or other metals (e.g., aluminium and tin)In addition, much modern research focuses on organic chemistry involving further organometallics, including the lanthanides, but especially the transition metals; (e.g., zinc, copper, palladium, nickel, cobalt, titanium and chromium)Finally, organic compounds form the basis of all earthly life and constitute a significant part of human endeavors in chemistry. The bonding patterns open to carbon, with its valence of four—formal single, double, and triple bonds, as well as various structures with delocalized electrons—make the array of organic compounds structurally diverse, and their range of applications enormous. They either form the basis of, or are important constituents of, many commercial products including pharmaceuticals; petrochemicals and products made from them (including lubricants, solvents, etc.); plastics; fuels and explosives; etc. As indicated, the study of organic chemistry overlaps with organometallic chemistry and biochemistry, but also with medicinal chemistry, polymer chemistry, as well as many aspects of materials science.