Chapter 3 Notes - Scarsdale Schools
... The atomic masses listed for the elements on the periodic table are weighted averages of their isotopes. That is why their masses are shown with decimal places (fractions of masses). The number and percentage of each isotope of an element is determined by a mass spectrograph. This instrument is ...
... The atomic masses listed for the elements on the periodic table are weighted averages of their isotopes. That is why their masses are shown with decimal places (fractions of masses). The number and percentage of each isotope of an element is determined by a mass spectrograph. This instrument is ...
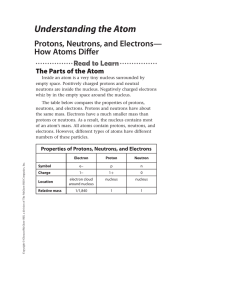
Understanding the Atom
... You can determine any one of these three quantities if you know the value of the other two quantities. For example, to determine the mass number of an atom, you must know the number of neutrons and the number of protons in the atom. The mass numbers of the isotopes of carbon are shown in the table a ...
... You can determine any one of these three quantities if you know the value of the other two quantities. For example, to determine the mass number of an atom, you must know the number of neutrons and the number of protons in the atom. The mass numbers of the isotopes of carbon are shown in the table a ...
File - Mrs. Dawson`s Classroom
... Example: Tin has 10 stable isotopes (the most of any element) ...
... Example: Tin has 10 stable isotopes (the most of any element) ...
Chapter 2
... atoms which carry a positive or negative electric charge. Common table salt (which has the chemical name of sodium chloride) is made of a large network of sodium ions, Na+ and chloride ions, Cl - ...
... atoms which carry a positive or negative electric charge. Common table salt (which has the chemical name of sodium chloride) is made of a large network of sodium ions, Na+ and chloride ions, Cl - ...
Atomic Structure - Miami East Schools
... simple whole number ratios to form compounds 5. Chemical reactions involve separating, combining, or rearranging atoms. ...
... simple whole number ratios to form compounds 5. Chemical reactions involve separating, combining, or rearranging atoms. ...
Atomic Structure Tick Sheet
... I know that all atoms of the same element have the SAME number of protons. I know that atoms of DIFFERENT elements have DIFFERENT numbers of protons. I know that the ATOMIC NUMBER of an atom is the BOTTOM NUMBER next to the symbol for the element in the Periodic Table. I know that the ATOMIC NUMBER ...
... I know that all atoms of the same element have the SAME number of protons. I know that atoms of DIFFERENT elements have DIFFERENT numbers of protons. I know that the ATOMIC NUMBER of an atom is the BOTTOM NUMBER next to the symbol for the element in the Periodic Table. I know that the ATOMIC NUMBER ...
Atoms and Elements
... All carbon atoms have 6 protons in their nuclei. The ______________________in the nucleus of an atom is called the atomic number. ________is the short-hand designation for the________________________. Because each element’s atoms have a unique number of protons, each element can be____________ ...
... All carbon atoms have 6 protons in their nuclei. The ______________________in the nucleus of an atom is called the atomic number. ________is the short-hand designation for the________________________. Because each element’s atoms have a unique number of protons, each element can be____________ ...
The Wizard Test Maker
... 2. Which of the following is NOT the same for isotopes of the same element? (A) Mass number (B) Atomic number (C) Number of protons (D) Number of valence electrons (E) Number of occupied electron shells in the ground state 3. Two isotopes of uranium are U-237 and U-238. Both would be expected to hav ...
... 2. Which of the following is NOT the same for isotopes of the same element? (A) Mass number (B) Atomic number (C) Number of protons (D) Number of valence electrons (E) Number of occupied electron shells in the ground state 3. Two isotopes of uranium are U-237 and U-238. Both would be expected to hav ...
Notes without questions - Department of Physics and Astronomy
... sugar molecule in its excited state (potential energy) until you release the energy via digestion, allowing the electron to “drop back” to a lower orbit (kinetic/chemical/heat energy) ...
... sugar molecule in its excited state (potential energy) until you release the energy via digestion, allowing the electron to “drop back” to a lower orbit (kinetic/chemical/heat energy) ...
Chemistry in Biology
... A. The force that holds atoms together is known as a chemical bond. • Electrons are directly involved in the formation of chemical bonds. -They travel around the nucleus of an atom in areas called energy levels. -Each energy levels has a specific number of electrons that it can hold at any time -The ...
... A. The force that holds atoms together is known as a chemical bond. • Electrons are directly involved in the formation of chemical bonds. -They travel around the nucleus of an atom in areas called energy levels. -Each energy levels has a specific number of electrons that it can hold at any time -The ...
NOTES: ATOMIC THEORY
... experimenting with cathode rays [a glowing beam which travels from the cathode (-) to the anode (+)]; his experiment is know as the cathode –ray tube experiment. He found that the e- was almost 2000 times lighter than a hydrogen atom, which was thought to be the lightest component of matter. (1911, ...
... experimenting with cathode rays [a glowing beam which travels from the cathode (-) to the anode (+)]; his experiment is know as the cathode –ray tube experiment. He found that the e- was almost 2000 times lighter than a hydrogen atom, which was thought to be the lightest component of matter. (1911, ...
An atom is the small unit of which all matter is made. It consists of
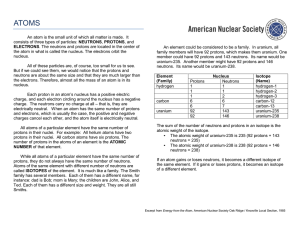
... atomic weight would be 238. 5. An isotope of an element gets part of its name from the total number of PROTONS and NEUTRONS in its nuclei. 6. If the isotope helium-4 has 2 protons and 2 neutrons, the isotope helium-6 would have 2 (two) protons and 4 (four) neutrons. 7. One element changes into anoth ...
... atomic weight would be 238. 5. An isotope of an element gets part of its name from the total number of PROTONS and NEUTRONS in its nuclei. 6. If the isotope helium-4 has 2 protons and 2 neutrons, the isotope helium-6 would have 2 (two) protons and 4 (four) neutrons. 7. One element changes into anoth ...
Unit Nuclear Chemistry
... verification for Einstein's equation E= MC2 › In 1928, he began to work on the acceleration of protons with Ernest Walton. › In 1932, they bombarded lithium with high energy neutrons, electrons and protons and succeeded in transmuting it into helium and other chemical elements. › This was one of the ...
... verification for Einstein's equation E= MC2 › In 1928, he began to work on the acceleration of protons with Ernest Walton. › In 1932, they bombarded lithium with high energy neutrons, electrons and protons and succeeded in transmuting it into helium and other chemical elements. › This was one of the ...
Welcome to Chemistry 1001
... integer ratio. Its properties are different to those of the component elements. (eg. water, alcohol) • A mixture has different elements or compounds mingled together. The properties of the components are maintained. (eg. beer, martini) ...
... integer ratio. Its properties are different to those of the component elements. (eg. water, alcohol) • A mixture has different elements or compounds mingled together. The properties of the components are maintained. (eg. beer, martini) ...
Unit 1 Notes (general chem review)
... may NOT indicate shape may or may not show the unbonded pairs of electrons ...
... may NOT indicate shape may or may not show the unbonded pairs of electrons ...
Structure of an Atom
... combine with atoms of other elements. All the noble gases, except helium, have 8 electrons in their outermost shell. Helium has only one shell which has 2 electrons. This condition is called duplet and is also stable. Thus, all the noble gases are chemically stable. They do not combine with atoms of ...
... combine with atoms of other elements. All the noble gases, except helium, have 8 electrons in their outermost shell. Helium has only one shell which has 2 electrons. This condition is called duplet and is also stable. Thus, all the noble gases are chemically stable. They do not combine with atoms of ...
Atoms - AJS Phyiscs and Chemistry
... electrons (use the group number to determine the number of valence electrons) 3) if an atom has more than 4 valence electrons, the additional electrons are paired. 4) elements in the same group will have identical dot diagrams since they have the same number of valence e- ...
... electrons (use the group number to determine the number of valence electrons) 3) if an atom has more than 4 valence electrons, the additional electrons are paired. 4) elements in the same group will have identical dot diagrams since they have the same number of valence e- ...
atom
... be. Atoms with electrons in higher energy levels have additional electron clouds of different shapes that also show where those electrons are likely to be. For more information, click here: http://regentsprep.org/Regents/physics/phys05/catomodel/cloud.htm ...
... be. Atoms with electrons in higher energy levels have additional electron clouds of different shapes that also show where those electrons are likely to be. For more information, click here: http://regentsprep.org/Regents/physics/phys05/catomodel/cloud.htm ...
Honors Mid-Term Review Sheet
... 81. How many lone pairs of electrons are in the Lewis dot structure for H2O? 82. Draw the Lewis dot structures for the following: CO, CO2, N2, and O2. 83. Define intermolecular forces and intramolecular forces. 84. Define London dispersion forces, dipole-dipole attractions, and hydrogen bonding. 85. ...
... 81. How many lone pairs of electrons are in the Lewis dot structure for H2O? 82. Draw the Lewis dot structures for the following: CO, CO2, N2, and O2. 83. Define intermolecular forces and intramolecular forces. 84. Define London dispersion forces, dipole-dipole attractions, and hydrogen bonding. 85. ...
I. The Atomic Concept:
... which deflect them from a straight course due to like-charge repulsion. A few alpha particles may have collided head-on with these centers, which must be dense or massive enough to bounce the alpha particles backward. Based on the small ratio of particles affected by the nuclei, he estimated the rat ...
... which deflect them from a straight course due to like-charge repulsion. A few alpha particles may have collided head-on with these centers, which must be dense or massive enough to bounce the alpha particles backward. Based on the small ratio of particles affected by the nuclei, he estimated the rat ...
Document
... new mass scale – STANDARDIZE atomic mass units (amu, dalton) 1 amu is about mass of a proton amu defined by mass of carbon-12: carbon 12: 6 protons and 6 neutrons define to have mass of exactly 12 amu ...
... new mass scale – STANDARDIZE atomic mass units (amu, dalton) 1 amu is about mass of a proton amu defined by mass of carbon-12: carbon 12: 6 protons and 6 neutrons define to have mass of exactly 12 amu ...
Interactive Notebook 2 for 2011-2012
... All atoms of any given element have the same numbers of protons (atomic number = Z) in their nucleus. Atoms are identified based on the number protons in the nucleus. The Periodic Table is organized in order of increasing atomic number. However, atoms of the same element may have different numbers o ...
... All atoms of any given element have the same numbers of protons (atomic number = Z) in their nucleus. Atoms are identified based on the number protons in the nucleus. The Periodic Table is organized in order of increasing atomic number. However, atoms of the same element may have different numbers o ...
14.1 Structure of the Atom
... internal structure and performed the first successful nuclear reaction. ...
... internal structure and performed the first successful nuclear reaction. ...
Chemistry 1 Lectures
... 2. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. 3. Compounds are composed of atoms of more than one element. The relative number of atoms of each element in a given compoun ...
... 2. All atoms of a given element are identical, having the same size, mass and chemical properties. The atoms of one element are different from the atoms of all other elements. 3. Compounds are composed of atoms of more than one element. The relative number of atoms of each element in a given compoun ...