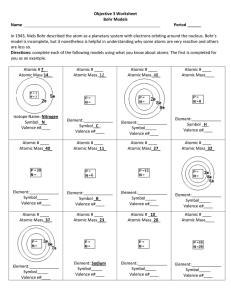
Ch # 5 Notes
... Ch # 5- Atoms, Molecules, Formulas and Subatomic Particles. Atom: An atom is the smallest particle of an element that can exist and still have properties of the element. Atomic Theory of matter: 1) All matter is made up of small particles called atoms.113 types. 2) Atoms of same element are similar ...
... Ch # 5- Atoms, Molecules, Formulas and Subatomic Particles. Atom: An atom is the smallest particle of an element that can exist and still have properties of the element. Atomic Theory of matter: 1) All matter is made up of small particles called atoms.113 types. 2) Atoms of same element are similar ...
Practice exam Part 3 Name 1) A Ca 2+ ion differs from a Ca0 atom in
... 2) Which particles are referred to as nucleons (subatomic particles located in the nucleus)? a) protons and neutrons c) neutrons, only b) protons and electrons d) neutrons and electrons 3) What is the mass number of an atom that contains 19 protons, 19 electrons, and 20 neutrons? a) 39 b) 19 c) ...
... 2) Which particles are referred to as nucleons (subatomic particles located in the nucleus)? a) protons and neutrons c) neutrons, only b) protons and electrons d) neutrons and electrons 3) What is the mass number of an atom that contains 19 protons, 19 electrons, and 20 neutrons? a) 39 b) 19 c) ...
Ch. 3.4 ppt. Isotopes
... As techniques for finding the masses of atoms has improved, we have learned that not all atoms of the same element are identical. Isotopes – • atoms of the same element that have different masses • vary in the number of neutrons they contain in the nucleus • almost all elements have more than one is ...
... As techniques for finding the masses of atoms has improved, we have learned that not all atoms of the same element are identical. Isotopes – • atoms of the same element that have different masses • vary in the number of neutrons they contain in the nucleus • almost all elements have more than one is ...
Chem Basics
... Model 2: Although an atom is defined by it’s protons, it’s the electrons that determine how it will behave. In a neutral atom (no charge), the number of protons and electrons is equal. The electrons move within ‘shells’ or ‘orbits’ about the nucleus; those in the outermost shell are called valence e ...
... Model 2: Although an atom is defined by it’s protons, it’s the electrons that determine how it will behave. In a neutral atom (no charge), the number of protons and electrons is equal. The electrons move within ‘shells’ or ‘orbits’ about the nucleus; those in the outermost shell are called valence e ...
ExamView - Chapter 4 Test.tst
... ____ 15. Which of the following is true about subatomic particles? a. Electrons are negatively charged and are the heaviest subatomic particle. b. Protons are positively charged and the lightest subatomic particle. c. Neutrons have no charge and are the lightest subatomic particle. d. The mass of a ...
... ____ 15. Which of the following is true about subatomic particles? a. Electrons are negatively charged and are the heaviest subatomic particle. b. Protons are positively charged and the lightest subatomic particle. c. Neutrons have no charge and are the lightest subatomic particle. d. The mass of a ...
Atom
... • Transmutation – conversion of an atom of one element to an atom of another element by spontaneous emission of radiation. • Induced Transmutation – nuclear reactions produced artificially by striking a nucleus with a high-velocity charged particle. ...
... • Transmutation – conversion of an atom of one element to an atom of another element by spontaneous emission of radiation. • Induced Transmutation – nuclear reactions produced artificially by striking a nucleus with a high-velocity charged particle. ...
Atomic Structure
... John Dalton’s Atomic Theory (1808) 1. Elements are made of indivisible particles called atoms. 2. Atoms of the same element are exactly alike; in particular, they have the same mass. 3. Compounds are formed by the joining of atoms of two or more elements in fixed, whole number ratios. e.g., 1:1, 2:1 ...
... John Dalton’s Atomic Theory (1808) 1. Elements are made of indivisible particles called atoms. 2. Atoms of the same element are exactly alike; in particular, they have the same mass. 3. Compounds are formed by the joining of atoms of two or more elements in fixed, whole number ratios. e.g., 1:1, 2:1 ...
Elements
... There are 7 elements that exist as diatomic molecules, you will simply need to find a way to memorize these. If you notice, all of the halogens fall in this category, and then hydrogen, nitrogen, and oxygen. You will also notice that 2 of these are not gases, make sure you do not for get to include ...
... There are 7 elements that exist as diatomic molecules, you will simply need to find a way to memorize these. If you notice, all of the halogens fall in this category, and then hydrogen, nitrogen, and oxygen. You will also notice that 2 of these are not gases, make sure you do not for get to include ...
Name Date Period______________ DIRECTED READING
... 11. The simplest atom is the __________________________ atom. It has one proton and one electron. 12. Neutrons in the atom’s nucleus keep two or more protons from moving apart. True or False? 13. If you build an atom using two protons, two neutrons, and two electrons, you have built an atom of _____ ...
... 11. The simplest atom is the __________________________ atom. It has one proton and one electron. 12. Neutrons in the atom’s nucleus keep two or more protons from moving apart. True or False? 13. If you build an atom using two protons, two neutrons, and two electrons, you have built an atom of _____ ...
atom
... g of oxygen and 9.60 g of carbon, and the other produces 21.6 g of oxygen and 8.10 g of carbon. Show that these results are consistent with the law of definite ...
... g of oxygen and 9.60 g of carbon, and the other produces 21.6 g of oxygen and 8.10 g of carbon. Show that these results are consistent with the law of definite ...
Unit 3 - Princeton High School
... _______________ _____ __________, that matter could not be created or destroyed. Then ___________ proposed, in his law of ____________ _____________, that the ratio of the masses of elements in any given compound is always the same. The law of _____________ ______________ , proposed soon after, stat ...
... _______________ _____ __________, that matter could not be created or destroyed. Then ___________ proposed, in his law of ____________ _____________, that the ratio of the masses of elements in any given compound is always the same. The law of _____________ ______________ , proposed soon after, stat ...
atoms
... • An experiment is an investigation with a control designed to test a hypothesis. • A theory is an explanation based on many observations and supported by the results of many investigations. ...
... • An experiment is an investigation with a control designed to test a hypothesis. • A theory is an explanation based on many observations and supported by the results of many investigations. ...
Unit Expectations – Polymers, Atom Model, Electron Configurations
... 1. P4.p2 Introduced: _______ Basic: _________ Mastered: _________ I can describe Elements as a class of substances composed of a single kind of atom, Compounds as two or more different elements chemically combined, and Mixtures as two or more different elements and/or compounds physically combined. ...
... 1. P4.p2 Introduced: _______ Basic: _________ Mastered: _________ I can describe Elements as a class of substances composed of a single kind of atom, Compounds as two or more different elements chemically combined, and Mixtures as two or more different elements and/or compounds physically combined. ...
Chem Midterm Review 2016
... Since these particles were smaller than atoms, but seemed to come from them, they must be subatomic parts. He concluded that electrons must be parts of atoms of all elements So, he discovered the first subatomic particle (electron), the atom is no longer indivisible, and developed the "plum pudding" ...
... Since these particles were smaller than atoms, but seemed to come from them, they must be subatomic parts. He concluded that electrons must be parts of atoms of all elements So, he discovered the first subatomic particle (electron), the atom is no longer indivisible, and developed the "plum pudding" ...
Structure of the Atom: Study Guide
... 2) Atoms are composed of two regions, what are they and what do they contain? 3) What does the Atomic Number tell you about an atom? 4) Which two particles add up to equal the mass number? 5) Solve this equation: ...
... 2) Atoms are composed of two regions, what are they and what do they contain? 3) What does the Atomic Number tell you about an atom? 4) Which two particles add up to equal the mass number? 5) Solve this equation: ...
Unit 3 - The Atom
... (4) Copper has an average atomic mass of 63.546 amu. It contains only two natural isotopes, which are Cu-63, with an isotope mass of 62.940 and Cu-65 with an isotope mass of 64.928. What are the percent of the two isotopes in naturally occurring copper? Avg. Atomic Mass = (%Cu-63 x Mass Cu-63) + (%C ...
... (4) Copper has an average atomic mass of 63.546 amu. It contains only two natural isotopes, which are Cu-63, with an isotope mass of 62.940 and Cu-65 with an isotope mass of 64.928. What are the percent of the two isotopes in naturally occurring copper? Avg. Atomic Mass = (%Cu-63 x Mass Cu-63) + (%C ...
1.What is the overall charge of an ion that has 12 protons
... What is the total mass of water formed when 8 grams of hydrogen reacts completely with 64 grams of ...
... What is the total mass of water formed when 8 grams of hydrogen reacts completely with 64 grams of ...
Periodic Table and Electrons
... Dimitri Mendeleev 1869, Professor of Chemistry at the University of Saint Petersburg (Leningrad). Mendeleev stated that the elements vary periodically (in cycles) according to their atomic masses. Mendeleev separated his elements and left spaces on his table in order for the periodicity to continue. ...
... Dimitri Mendeleev 1869, Professor of Chemistry at the University of Saint Petersburg (Leningrad). Mendeleev stated that the elements vary periodically (in cycles) according to their atomic masses. Mendeleev separated his elements and left spaces on his table in order for the periodicity to continue. ...
chemistry notes: atomic structure
... A. early theories and ideas, pro and con 1) Democritus of Abdera (460-370 B.C.): first atomic theory of matter • “atoma” / “atomos”—indivisible, indestructible particles in matter 2) Aristotle (384-322 B.C.): did not believe in atoms • “hyle”— continuous state of all matter • His theory was widely a ...
... A. early theories and ideas, pro and con 1) Democritus of Abdera (460-370 B.C.): first atomic theory of matter • “atoma” / “atomos”—indivisible, indestructible particles in matter 2) Aristotle (384-322 B.C.): did not believe in atoms • “hyle”— continuous state of all matter • His theory was widely a ...
The format of this test is MULTIPLE CHOICE
... content in each unit covered and allow you practice with the content from this semester. It is not intended to address any specific test question on the final exam. Completion of this packet does not guarantee success on the Final Exam, but practicing with the content is a great idea. Unit 1: Scient ...
... content in each unit covered and allow you practice with the content from this semester. It is not intended to address any specific test question on the final exam. Completion of this packet does not guarantee success on the Final Exam, but practicing with the content is a great idea. Unit 1: Scient ...
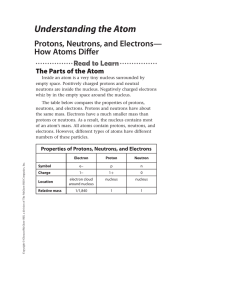
Understanding the Atom
... Look at the periodic table on the inside back cover of this book. Notice that more than 115 different elements have been identified. Recall that an element is a substance made from atoms that all have the same number of protons. For example, the element carbon is made from atoms that all have six pr ...
... Look at the periodic table on the inside back cover of this book. Notice that more than 115 different elements have been identified. Recall that an element is a substance made from atoms that all have the same number of protons. For example, the element carbon is made from atoms that all have six pr ...
Chapter 2 ppt
... and electricity; have a metallic shine; tend to lose electrons during reactions ...
... and electricity; have a metallic shine; tend to lose electrons during reactions ...