Do not forget to study your polyatomic ions! Honors Chemistry
... 18. The number of significant figures in the measured value 0.032 0 g is a. 2. b. 3. c. 4. d. 5. 19. Which orbitals can be modeled as dumbbell shaped? a. s b. p c. d d. f 20. The most massive particle in an atom is the a. proton. b. neutron. c. electron. d. None of the above 21. The molar mass of an ...
... 18. The number of significant figures in the measured value 0.032 0 g is a. 2. b. 3. c. 4. d. 5. 19. Which orbitals can be modeled as dumbbell shaped? a. s b. p c. d d. f 20. The most massive particle in an atom is the a. proton. b. neutron. c. electron. d. None of the above 21. The molar mass of an ...
Atom
... Elements are different because they contain different number of protons. Atomic number – of an element is the number of protons in the nucleus of an atom of that element. Example – all hydrogen atoms have 1 proton and the atomic number of hydrogen is 1. The atomic number identifies an element. ...
... Elements are different because they contain different number of protons. Atomic number – of an element is the number of protons in the nucleus of an atom of that element. Example – all hydrogen atoms have 1 proton and the atomic number of hydrogen is 1. The atomic number identifies an element. ...
History of Atomic Models
... Electron shell- the set of orbitals within the same principle energy level (n). Azimuthal Quantum Number (l- a lowercase letter L), describes the sublevels of each principle energy level. Sublevels- symmetrical shapes that surround the nucleus represented by the letters s, p, d, and f. Subshell- the ...
... Electron shell- the set of orbitals within the same principle energy level (n). Azimuthal Quantum Number (l- a lowercase letter L), describes the sublevels of each principle energy level. Sublevels- symmetrical shapes that surround the nucleus represented by the letters s, p, d, and f. Subshell- the ...
Period:______ Table Number
... 46. The smallest particle of any element that you can have which still possesses all of the physical and chemical properties of that element is a single ATOM of that element. P. 10, VCR: Atoms and Molecules 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said ...
... 46. The smallest particle of any element that you can have which still possesses all of the physical and chemical properties of that element is a single ATOM of that element. P. 10, VCR: Atoms and Molecules 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said ...
c. Section 3.3 Elements and the Periodic Table
... electrons it can hold 1st shell up to 2 electrons 2nd shell up to 8 electrons 3rd shell up to 8 electrons 4th shell up to 18 electrons • This pattern of electrons is followed by all atoms, although not all atoms have that many electrons. ...
... electrons it can hold 1st shell up to 2 electrons 2nd shell up to 8 electrons 3rd shell up to 8 electrons 4th shell up to 18 electrons • This pattern of electrons is followed by all atoms, although not all atoms have that many electrons. ...
U1 Atoms, Periodic Table, Variables, Conversions Unit 1
... 17. A certain atom has 20 electrons, 21 neutrons, and 20 protons. What is the atomic mass of the ...
... 17. A certain atom has 20 electrons, 21 neutrons, and 20 protons. What is the atomic mass of the ...
1.1 Early Ideas of the Atom
... indication that science is a waste of time? 1. Match the person, or group of people, with their role in the development of chemistry. ...
... indication that science is a waste of time? 1. Match the person, or group of people, with their role in the development of chemistry. ...
The Atom - davis.k12.ut.us
... and discovered a high concentration of mass and positive charge at the nucleus of the atom with the very light electrons distributed ...
... and discovered a high concentration of mass and positive charge at the nucleus of the atom with the very light electrons distributed ...
atom
... Dalton’s Atomic Theory All matter is made of tiny indivisible particles called atoms. Atoms of the same element are identical, those of different atoms are different. Atoms of different elements combine in whole number ratios to form compounds. Chemical reactions involve the rearrangement o ...
... Dalton’s Atomic Theory All matter is made of tiny indivisible particles called atoms. Atoms of the same element are identical, those of different atoms are different. Atoms of different elements combine in whole number ratios to form compounds. Chemical reactions involve the rearrangement o ...
Unit 3-The Atom Chapter Packet
... ____________, that matter could not be created or destroyed. Then ___________ proposed, in his law of _________ _________, that the ratio of the masses of elements in any given compound is always the same. The law of ____________ ___________ , proposed soon after, states that the masses of one eleme ...
... ____________, that matter could not be created or destroyed. Then ___________ proposed, in his law of _________ _________, that the ratio of the masses of elements in any given compound is always the same. The law of ____________ ___________ , proposed soon after, states that the masses of one eleme ...
AtomicStructure_Peri..
... the number of electron energy levels remain the same. Positive protons and negative electrons attract each other. This increase in the number of protons causes a greater attraction between the nucleus and the electrons in the atom. As protons are added, electrons are pulled closer the nucleus giving ...
... the number of electron energy levels remain the same. Positive protons and negative electrons attract each other. This increase in the number of protons causes a greater attraction between the nucleus and the electrons in the atom. As protons are added, electrons are pulled closer the nucleus giving ...
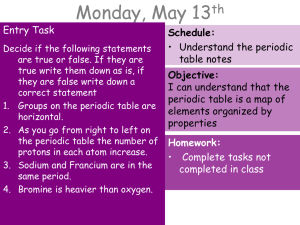
Entry Task
... • Nonmetals– elements on the right of the periodic table – Properties are opposite of metals – Their properties vary more from element to element than the properties of metals – All are gas in their natural state at room temperature except for bromine which is a liquid – Poor conductors of heat and ...
... • Nonmetals– elements on the right of the periodic table – Properties are opposite of metals – Their properties vary more from element to element than the properties of metals – All are gas in their natural state at room temperature except for bromine which is a liquid – Poor conductors of heat and ...
Chapter 2 Atoms and Elements
... Now using the mass of Hydrogen and Oxygen to show these results are consistent with the law of definite proportion ...
... Now using the mass of Hydrogen and Oxygen to show these results are consistent with the law of definite proportion ...
The Atom
... • Most of the atomic mass is present in the nucleus because the mass of the electron is almost equal to zero. • Mass of a proton = mass of a neutron= 1a.m.u • The atom has no charge (neutral) because number of protons = number of electrons. ...
... • Most of the atomic mass is present in the nucleus because the mass of the electron is almost equal to zero. • Mass of a proton = mass of a neutron= 1a.m.u • The atom has no charge (neutral) because number of protons = number of electrons. ...
TEK 8.5A: Atomic Structure
... of an element with different number of neutrons are called isotopes of that element. Atoms of a particular element can thus have different atomic masses. ...
... of an element with different number of neutrons are called isotopes of that element. Atoms of a particular element can thus have different atomic masses. ...
STAAR Science Tutorial 09 TEK 8.5A: Atomic Structure
... of an element with different number of neutrons are called isotopes of that element. Atoms of a particular element can thus have different atomic masses. ...
... of an element with different number of neutrons are called isotopes of that element. Atoms of a particular element can thus have different atomic masses. ...
File - Flipped Out Science with Mrs. Thomas!
... of an element with different number of neutrons are called isotopes of that element. Atoms of a particular element can thus have different atomic masses. ...
... of an element with different number of neutrons are called isotopes of that element. Atoms of a particular element can thus have different atomic masses. ...
atom
... Dalton’s Atomic Theory All matter is made of tiny indivisible particles called atoms. Atoms of the same element are identical, those of different atoms are different. Atoms of different elements combine in whole number ratios to form compounds. Chemical reactions involve the rearrangement o ...
... Dalton’s Atomic Theory All matter is made of tiny indivisible particles called atoms. Atoms of the same element are identical, those of different atoms are different. Atoms of different elements combine in whole number ratios to form compounds. Chemical reactions involve the rearrangement o ...
1 | Page Chemistry Lecture #19: Atomic Number, Isotopes, and
... For now, ignore the 14.0067 (I’ll explain what this number is in another lecture). The number 7 is the atomic number of nitrogen. Thus, nitrogen has 7 protons in the nucleus. In a neutral atom, the number of protons in the nucleus is equal to the number of electrons. ...
... For now, ignore the 14.0067 (I’ll explain what this number is in another lecture). The number 7 is the atomic number of nitrogen. Thus, nitrogen has 7 protons in the nucleus. In a neutral atom, the number of protons in the nucleus is equal to the number of electrons. ...
atomic number
... Determining the Identity of an Element Each element on the Periodic Table is made of only one kind of atom. The number of protons determines an element’s IDENTITY. The number of protons is represented by the ...
... Determining the Identity of an Element Each element on the Periodic Table is made of only one kind of atom. The number of protons determines an element’s IDENTITY. The number of protons is represented by the ...
SCIENCE LONG TEST
... atoms were small, hard particles made of the same material but of different shapes and sizes there were an infinite number of these atoms and they were constantly in motion atoms had the ability to combine with other atoms atoms could no longer be divided into smaller particles The early ideas about ...
... atoms were small, hard particles made of the same material but of different shapes and sizes there were an infinite number of these atoms and they were constantly in motion atoms had the ability to combine with other atoms atoms could no longer be divided into smaller particles The early ideas about ...
Element A pure substance made of only one type of atom which
... The last shell can also hold eight, but holds six as there are only six left. There are three shells altogether (which is why sulphur is in period 3). There are six electrons in the outermost shell (which is why sulphur is in group VI). Atoms are always trying to end up with a full outer shell ...
... The last shell can also hold eight, but holds six as there are only six left. There are three shells altogether (which is why sulphur is in period 3). There are six electrons in the outermost shell (which is why sulphur is in group VI). Atoms are always trying to end up with a full outer shell ...
Unit 03 Packet - Whitwell High School
... nucleus of an atom. The atom is __________ because this is also the number of __________ charged __________ in the atom. 2. The mass number tells the total number of________ and _________ in the nucleus of an atom. These particles collectively are called ___________ since both are located in the nuc ...
... nucleus of an atom. The atom is __________ because this is also the number of __________ charged __________ in the atom. 2. The mass number tells the total number of________ and _________ in the nucleus of an atom. These particles collectively are called ___________ since both are located in the nuc ...
Chapter 4: Chemical Reactions Elements can be characterized as
... Table 4-11 (List of common cations and anions) Binary molecular compounds (Mostly two nonmetals bonded together) Use Greek and Latin prefixes instead of Roman numerals and suffixes. Examples: SO2 – sulfur dioxide; SO3 – sulfur trioxide; As4O6 – tetraarsenic hexoxide Learn the common prefixes (pg. 14 ...
... Table 4-11 (List of common cations and anions) Binary molecular compounds (Mostly two nonmetals bonded together) Use Greek and Latin prefixes instead of Roman numerals and suffixes. Examples: SO2 – sulfur dioxide; SO3 – sulfur trioxide; As4O6 – tetraarsenic hexoxide Learn the common prefixes (pg. 14 ...