Atoms and Molecules
... Note: In 1869, Dmitri Mendeleyev was credited with putting together the Periodic Table of Elements. He listed all of the known elements and grouped them together based on their properties. Mendeleyev was able to organize the table in its present form even though many of the elements hadn’t been disc ...
... Note: In 1869, Dmitri Mendeleyev was credited with putting together the Periodic Table of Elements. He listed all of the known elements and grouped them together based on their properties. Mendeleyev was able to organize the table in its present form even though many of the elements hadn’t been disc ...
Ch 3 Sec 3 Highlighted
... and tells you how many protons it has, and how many electrons it has. If you want to know which element has atomic number 47 look at the periodic table it is silver, Ag. All silver atoms contain 47 protons in their nuclei. Because atoms are neutral, we know from the atomic number that all silver ato ...
... and tells you how many protons it has, and how many electrons it has. If you want to know which element has atomic number 47 look at the periodic table it is silver, Ag. All silver atoms contain 47 protons in their nuclei. Because atoms are neutral, we know from the atomic number that all silver ato ...
Chemistry
... 1) The smallest particle of an element that retains the properties of that element is the: a) cell b) proton c) electron d) neutron e) none of the above 2) Which of the following is not a part of Dalton’s atomic theory? a) All elements are composed of atoms. b) The positive charge of the atom is loc ...
... 1) The smallest particle of an element that retains the properties of that element is the: a) cell b) proton c) electron d) neutron e) none of the above 2) Which of the following is not a part of Dalton’s atomic theory? a) All elements are composed of atoms. b) The positive charge of the atom is loc ...
Atomic Structure Worksheet
... b. An orbital can contain a maximum of two electrons. c. An electron cloud represents all the orbitals in an atom. d. An atom’s lowest energy level has only one orbital. ____ 16. The glowing of a neon light is caused by electrons emitting energy as they a. move from lower to higher energy c. move fr ...
... b. An orbital can contain a maximum of two electrons. c. An electron cloud represents all the orbitals in an atom. d. An atom’s lowest energy level has only one orbital. ____ 16. The glowing of a neon light is caused by electrons emitting energy as they a. move from lower to higher energy c. move fr ...
5 Early Atomic Theory and Structure Chapter Outline Early Theories
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
... 3. Atoms of different elements differ in their mass and size. 4. Compounds are formed by combining two or more atoms of different elements. 5. Atoms combine to form compounds in simple whole number ratios. ...
Atoms and Molecules
... Q: How is the Periodic Table arranged with respect to the number of protons an atom has? A: The Periodic Table is arranged in increasing Atomic Number, which corresponds to an increasing number of protons in each element. Q: How many elements are there? A: There are 117 known elements. 90 of them ar ...
... Q: How is the Periodic Table arranged with respect to the number of protons an atom has? A: The Periodic Table is arranged in increasing Atomic Number, which corresponds to an increasing number of protons in each element. Q: How many elements are there? A: There are 117 known elements. 90 of them ar ...
Unit #3: ATOMIC STRUCTURE - Miss Virga`s Chemistry Class
... Base your answers to questions 12 through 14 on the information and diagram below. One model of the atom states that atoms are tiny particles composed of a uniform mixture of positive and negative charges. Scientists conducted an experiment where alpha particles were aimed at a thin layer of gold at ...
... Base your answers to questions 12 through 14 on the information and diagram below. One model of the atom states that atoms are tiny particles composed of a uniform mixture of positive and negative charges. Scientists conducted an experiment where alpha particles were aimed at a thin layer of gold at ...
Chapter 2 Atoms and Elements
... atoms lost the same negative particles, the resulting positive atoms (ions) were different from atom to atom. The simplest (lightest) of these positive atoms was hydrogen, which was later shown to be a single proton (p+), the positively charged objects in the atom. Further experiments showed that th ...
... atoms lost the same negative particles, the resulting positive atoms (ions) were different from atom to atom. The simplest (lightest) of these positive atoms was hydrogen, which was later shown to be a single proton (p+), the positively charged objects in the atom. Further experiments showed that th ...
Atoms and Elements Practice Test Chemistry
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement below accurately describes the 7) Which statement below is NOT consistent with the contributions of Democritus? nuclear theory of the atom as proposed by Rutherford? A) created t ...
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement below accurately describes the 7) Which statement below is NOT consistent with the contributions of Democritus? nuclear theory of the atom as proposed by Rutherford? A) created t ...
1. Atomic Structure
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
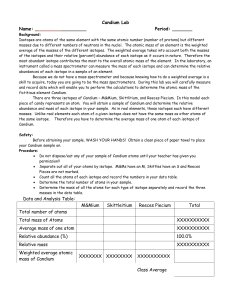
Candium Lab - OCPS TeacherPress
... of the isotopes and their relative (percent) abundance of each isotope as it occurs in nature. Therefore the most abundant isotope contributes the most to the overall atomic mass of the element. In the laboratory, an instrument called a mass spectrometer can measure the mass of each isotope and can ...
... of the isotopes and their relative (percent) abundance of each isotope as it occurs in nature. Therefore the most abundant isotope contributes the most to the overall atomic mass of the element. In the laboratory, an instrument called a mass spectrometer can measure the mass of each isotope and can ...
Chapter 3 Atoms and Elements
... In Dalton's atomic theory, atoms: • are tiny particles of matter • of an element are similar to each other and different from other elements • of two or more different elements combine to form compounds • are rearranged to form new combinations in a chemical reaction ...
... In Dalton's atomic theory, atoms: • are tiny particles of matter • of an element are similar to each other and different from other elements • of two or more different elements combine to form compounds • are rearranged to form new combinations in a chemical reaction ...
Powerpoint covering atomic structure and isotopes
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
... Atoms are very small – they are about 0.00000001 cm wide. Think about the thickness of a crisp. The number of atoms you would need to stack up to make the thickness of a crisp, is approximately the same number of crisps you would need to stack up to make the height of Mount Everest! ...
ch03 - earthjay science
... alpha particle (37): A particle equivalent to the nucleus of a helium atom, emitted from an atomic nucleus during radioactive decay. Archean Eon (30): Pertaining to the division of Precambrian beginning 3.8 billion years ago and ending 2.5 billion years ago. atom (36): The smallest particle of matte ...
... alpha particle (37): A particle equivalent to the nucleus of a helium atom, emitted from an atomic nucleus during radioactive decay. Archean Eon (30): Pertaining to the division of Precambrian beginning 3.8 billion years ago and ending 2.5 billion years ago. atom (36): The smallest particle of matte ...
STRUCTURE OF THE ATOM
... This model is also called Plum Pudding Model or Apple Pie Model. JJ Thomson gave the first model of the atom. In this model electrons were uniformly distributed in the entire atom. The mass of the atom was supposed to be uniformly ...
... This model is also called Plum Pudding Model or Apple Pie Model. JJ Thomson gave the first model of the atom. In this model electrons were uniformly distributed in the entire atom. The mass of the atom was supposed to be uniformly ...
Directed Reading
... ______ 29. What is the atomic number of an element? a. the number of neutrons in the nucleus of the atom b. the number of protons and neutrons in the nucleus of the atom c. the number of protons in the nucleus of the atom d. the number of electrons in the nucleus of an atom ______ 30. An uncharged a ...
... ______ 29. What is the atomic number of an element? a. the number of neutrons in the nucleus of the atom b. the number of protons and neutrons in the nucleus of the atom c. the number of protons in the nucleus of the atom d. the number of electrons in the nucleus of an atom ______ 30. An uncharged a ...
Chapter 7 Periodic Properties of the Elements
... • Successive shells are larger than the previous shells. • Electrons in successive shells spend more of their time further away from the nucleus. • Electrons in successive shells are partly “shielded” from the attractive forces (of protons) by the electrons in the previous shells. • This shielding e ...
... • Successive shells are larger than the previous shells. • Electrons in successive shells spend more of their time further away from the nucleus. • Electrons in successive shells are partly “shielded” from the attractive forces (of protons) by the electrons in the previous shells. • This shielding e ...
Chapter 1 The Periodic Table - Beck-Shop
... Transition metals have high melting temperatures, are hard and dense and are highly reactive. D. Most transition metals can form more than one oxidation state. Question 19 The properties of the elements of the third period vary as one goes across the period from Na to Ar. Which one of the following ...
... Transition metals have high melting temperatures, are hard and dense and are highly reactive. D. Most transition metals can form more than one oxidation state. Question 19 The properties of the elements of the third period vary as one goes across the period from Na to Ar. Which one of the following ...
Chapter 18: Properties of Atoms and the Periodic Table
... ■ Interpret the average atomic mass of an element. ...
... ■ Interpret the average atomic mass of an element. ...
Chapter 6 ppt
... The Parts of an Atom, continued • The charges of protons and electrons are opposite but equal, so the charges cancel out. If the numbers of electrons and protons become unequal, the atom becomes a charged particle called an ion. • The SI unit that is used to express the mass of a particle in an atom ...
... The Parts of an Atom, continued • The charges of protons and electrons are opposite but equal, so the charges cancel out. If the numbers of electrons and protons become unequal, the atom becomes a charged particle called an ion. • The SI unit that is used to express the mass of a particle in an atom ...
Mass Number, A
... “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-‐inch shell at a piece of 2ssue paper and it came back ...
... “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-‐inch shell at a piece of 2ssue paper and it came back ...
2.1 Imaging and Moving Individual Atoms
... They have been rounded off to show the uncertainty in the measurement. For example, if you measure the mass of a certain object on a balance and found that it gives a mass of 25.0125 g, 25.013, 25.01 g 25.0 g or 25 g with a decimal place rounded off to a place at right depending on the decreasing ac ...
... They have been rounded off to show the uncertainty in the measurement. For example, if you measure the mass of a certain object on a balance and found that it gives a mass of 25.0125 g, 25.013, 25.01 g 25.0 g or 25 g with a decimal place rounded off to a place at right depending on the decreasing ac ...
Chapter 3—Time and Geology
... atom (34): The smallest particle of matter that can exist as a chemical element. atomic mass (34): A quantity essentially equivalent to the number of neutrons plus the number of protons in an atomic nucleus. atomic number (34): The number of protons in the nuclei of atoms of a particular element. An ...
... atom (34): The smallest particle of matter that can exist as a chemical element. atomic mass (34): A quantity essentially equivalent to the number of neutrons plus the number of protons in an atomic nucleus. atomic number (34): The number of protons in the nuclei of atoms of a particular element. An ...