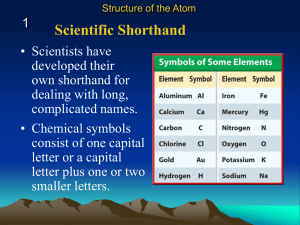
Element A pure substance made of only one type of atom which
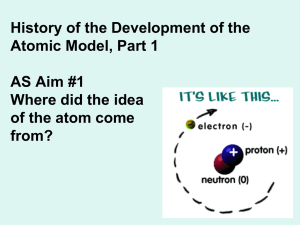
... Atoms consist of electrons that surround a nucleus. The nucleus contains protons and neutrons. NEUtrons are NEUtral (have a neutral charge) PROtons are POsitively charged (have a charge of +1) Electrons are negatively charged (have a charge of -1) The mass number (aka nucleon number) is the sum of t ...
... Atoms consist of electrons that surround a nucleus. The nucleus contains protons and neutrons. NEUtrons are NEUtral (have a neutral charge) PROtons are POsitively charged (have a charge of +1) Electrons are negatively charged (have a charge of -1) The mass number (aka nucleon number) is the sum of t ...
Notes powerpoint
... • For example, four out of five atoms of boron are boron-11, and one out of five is boron-10. • To find the weighted-average or the average atomic mass of boron, you would solve the following equation: ...
... • For example, four out of five atoms of boron are boron-11, and one out of five is boron-10. • To find the weighted-average or the average atomic mass of boron, you would solve the following equation: ...
4.1 Studying Atoms
... • Democritus (460-370 B.C.): was the first to use the word “atomos”( which meant atoms); stated that matter is composed of atoms; atoms are indestructible and indivisible ...
... • Democritus (460-370 B.C.): was the first to use the word “atomos”( which meant atoms); stated that matter is composed of atoms; atoms are indestructible and indivisible ...
Parts per million
... *Calculate the amount of substance in a solution of known concentration *Use chemical equations to calculate reacting masses *Use chemical equations to calculate volumes of gases *Use chemical equations to calculate volumes of gases and vice versa using the concepts of amount of substance and molar ...
... *Calculate the amount of substance in a solution of known concentration *Use chemical equations to calculate reacting masses *Use chemical equations to calculate volumes of gases *Use chemical equations to calculate volumes of gases and vice versa using the concepts of amount of substance and molar ...
4.01 Evolution of the Atomic Theory
... String Theory String theory is an active research framework in particle physics that attempts to reconcile quantum mechanics and general relativity general relativity.[1] It is a contender for the theory of everything (TOE), a manner of describing the known fundamental forces and matter in a mathem ...
... String Theory String theory is an active research framework in particle physics that attempts to reconcile quantum mechanics and general relativity general relativity.[1] It is a contender for the theory of everything (TOE), a manner of describing the known fundamental forces and matter in a mathem ...
Atomic Structure
... accounting for more than 99% of the mass of the atom The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
... accounting for more than 99% of the mass of the atom The negatively charged electrons are small and have a relatively small mass but occupy a large volume of space outside the nucleus ...
Chapter 10
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
Chapter 2 - Bruder Chemistry
... called atoms Atoms of the same element are identical. The atoms of anyone element are different from those of any other element Atoms of different elements can physically mix together or can chemically combine w/ one another in simple whole-number ratios to form compounds Chemical reactions occur wh ...
... called atoms Atoms of the same element are identical. The atoms of anyone element are different from those of any other element Atoms of different elements can physically mix together or can chemically combine w/ one another in simple whole-number ratios to form compounds Chemical reactions occur wh ...
Is Kr the symbol for Kryptonite?
... The Elements Formulas of compounds • Write the formulas for the following compounds: – A phosphorus atom bonded to three chlorine atoms – A molecule containing two boron atoms and six hydrogen atoms – A compound containing one calcium atom for every two chlorine atoms – Four hydrogen atoms bond ...
... The Elements Formulas of compounds • Write the formulas for the following compounds: – A phosphorus atom bonded to three chlorine atoms – A molecule containing two boron atoms and six hydrogen atoms – A compound containing one calcium atom for every two chlorine atoms – Four hydrogen atoms bond ...
1.3 PPT - gessramsey
... mass and size, but they are different in mass and size from the atoms of other elements. Compounds are created when atoms of different elements link together in definite ...
... mass and size, but they are different in mass and size from the atoms of other elements. Compounds are created when atoms of different elements link together in definite ...
atom`s - Hauppauge School District
... stable electron configuration of eight • Determine how many electrons are gained or lost • Write the new electron configuration Element ...
... stable electron configuration of eight • Determine how many electrons are gained or lost • Write the new electron configuration Element ...
presentation source
... -elements are composed of small particles -- atoms -all atoms of an element are identical -atoms are not created or destroyed chemically -compounds formed by chemical combination of two or more elements -a given compound has same relative number & type of atoms (law of constant composition) -atoms r ...
... -elements are composed of small particles -- atoms -all atoms of an element are identical -atoms are not created or destroyed chemically -compounds formed by chemical combination of two or more elements -a given compound has same relative number & type of atoms (law of constant composition) -atoms r ...
The Atom Notes
... The Atom and The Atomic Structure •The Atomic - Molecular Theory of Matter states that all matter is composed of small, fast moving particles called atoms. These atoms can join together to form molecules. ...
... The Atom and The Atomic Structure •The Atomic - Molecular Theory of Matter states that all matter is composed of small, fast moving particles called atoms. These atoms can join together to form molecules. ...
File
... of an element have the same atomic number but different mass numbers because they have different numbers of neutrons. For example Carbon 14 is a radioactive isotope. Usually, carbon has a mass number of 12, but Carbon 14 has a mass number of 14 due to the 2 extra neutrons. 6 protons + 8 neutrons ...
... of an element have the same atomic number but different mass numbers because they have different numbers of neutrons. For example Carbon 14 is a radioactive isotope. Usually, carbon has a mass number of 12, but Carbon 14 has a mass number of 14 due to the 2 extra neutrons. 6 protons + 8 neutrons ...
atom
... identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds In chemical reactions, atoms are comb ...
... identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties. Atoms cannot be subdivided, created, or destroyed. Atoms of different elements combine in simple whole-number ratios to form chemical compounds In chemical reactions, atoms are comb ...
The Building Blocks of Matter
... • Just as we have the atomic # (# of protons) we have atomic mass #: Atomic mass # (A) - the # of protons + neutrons in an atom. • Because the bulk of the atom’s mass is provided by the protons and neutrons, we only consider their masses when calculating the atomic mass #. – Ex: O has 8 protons and ...
... • Just as we have the atomic # (# of protons) we have atomic mass #: Atomic mass # (A) - the # of protons + neutrons in an atom. • Because the bulk of the atom’s mass is provided by the protons and neutrons, we only consider their masses when calculating the atomic mass #. – Ex: O has 8 protons and ...
Atomic Theory PPT
... Atomic Mass o The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The a ...
... Atomic Mass o The atomic mass of an element represents the average mass of all the isotopes found in nature. No element exists with only one possible isotope. Hydrogen has the smallest number of isotopes: 1H protium, 2H deuterium, 3H tritium. Its atomic mass is 1.0079 amu (atomic mass units). The a ...
How many protons, electrons and neutrons are in an atom of krypton
... In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. Electrons are arranged around atoms in a special way. If you need to know how the electrons are arranged around an atom, take a look at the 'How do I read an electron configuration table?' page. An atom can ga ...
... In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. Electrons are arranged around atoms in a special way. If you need to know how the electrons are arranged around an atom, take a look at the 'How do I read an electron configuration table?' page. An atom can ga ...
Slide 1 - Herricks
... 4. Balance the elements one at a time by using coefficients. When no coefficient is written, it is assumed to be 1. Begin by balancing elements that appear only once on each side of the equation. Never balance an equation by changing the subscripts in a chemical formula. Each substance has only one ...
... 4. Balance the elements one at a time by using coefficients. When no coefficient is written, it is assumed to be 1. Begin by balancing elements that appear only once on each side of the equation. Never balance an equation by changing the subscripts in a chemical formula. Each substance has only one ...
Atomic Structure - Hudson City School District
... • (help dental decay) But too much can cause fluorosis (white chalky buildup) ...
... • (help dental decay) But too much can cause fluorosis (white chalky buildup) ...
File - Mr. L`s Room
... 10. Show the molecules of solids, liquids, and gases. Use arrows to show their relative energy level. ...
... 10. Show the molecules of solids, liquids, and gases. Use arrows to show their relative energy level. ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.