the Note
... prediction called a hypothesis Planned experiments are carried out that are fair Data is collected and the results are analysed A conclusion is made and tested against the hypothesis – sometimes the hypothesis is confirmed but most times completely unexpected results are obtained. ...
... prediction called a hypothesis Planned experiments are carried out that are fair Data is collected and the results are analysed A conclusion is made and tested against the hypothesis – sometimes the hypothesis is confirmed but most times completely unexpected results are obtained. ...
File
... 3) Used by Rutherford in his experiment; made of two protons and two neutrons 4) The paths in which electrons circle the nucleus according to the Bohr model 5) The positive particle in the nucleus of an atom 6) The tiny positive core of an atom; contains protons and neutrons 7) Formed the atomic the ...
... 3) Used by Rutherford in his experiment; made of two protons and two neutrons 4) The paths in which electrons circle the nucleus according to the Bohr model 5) The positive particle in the nucleus of an atom 6) The tiny positive core of an atom; contains protons and neutrons 7) Formed the atomic the ...
Atoms, Molecules, and Ions Chemistry Timeline #1
... Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements ...
... Ernest Rutherford: Existence of the nucleus, and its relative size Meitner & Fermi: Sustained nuclear fission Ernest Lawrence: The cyclotron and trans-uranium elements ...
Chapter 3 Atoms and Elements
... Isotopes of Some Elements and Their Atomic Mass Most elements have two or more isotopes that contribute to the atomic mass of that element. ...
... Isotopes of Some Elements and Their Atomic Mass Most elements have two or more isotopes that contribute to the atomic mass of that element. ...
Atoms
... be broken down (atoms) same as Democritus 2. All atoms of the same element are identical in size, mass and properties. Atoms of different elements are different in ...
... be broken down (atoms) same as Democritus 2. All atoms of the same element are identical in size, mass and properties. Atoms of different elements are different in ...
What are elements?
... • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
... • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
Document
... 2. Which of the following is not an element? 1. oxygen 2. sodium chloride 3. hydrogen 4. nitrogen ...
... 2. Which of the following is not an element? 1. oxygen 2. sodium chloride 3. hydrogen 4. nitrogen ...
Word - The Chemistry Book
... plate; and the rest went through the magnetic field without deflection. Thus, there were three types of radioactivity: alpha particles (+), beta particles (-) and gamma rays (neutral). By performing other experiments and using this information, Rutherford created an atomic model different from Thoms ...
... plate; and the rest went through the magnetic field without deflection. Thus, there were three types of radioactivity: alpha particles (+), beta particles (-) and gamma rays (neutral). By performing other experiments and using this information, Rutherford created an atomic model different from Thoms ...
Avg. Atomic Mass - Greer Middle College
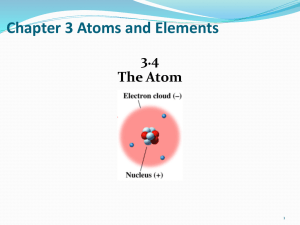
... planets circle the sun. These orbits, or energy levels, are located at certain distances from the nucleus. ...
... planets circle the sun. These orbits, or energy levels, are located at certain distances from the nucleus. ...
Chapter 4: The Structure of the Atom
... 1) One of the four naturally occurring isotopes of chromium has a mass number of 53. Determine the number of protons, electrons, and neutrons in an atom of this isotope and write its symbol. 2) The other three naturally occurring isotopes of chromium have mass number of 50, 52, and 54. Describe ...
... 1) One of the four naturally occurring isotopes of chromium has a mass number of 53. Determine the number of protons, electrons, and neutrons in an atom of this isotope and write its symbol. 2) The other three naturally occurring isotopes of chromium have mass number of 50, 52, and 54. Describe ...
File
... weight is multiplied by its percent abundance (expressed as a decimal). Then, add the results together and round off to an appropriate number of significant figures. This is the solution for carbon: ...
... weight is multiplied by its percent abundance (expressed as a decimal). Then, add the results together and round off to an appropriate number of significant figures. This is the solution for carbon: ...
Say Thanks to the Authors Click http://www.ck12.org/saythanks (No
... The graphite in your pencil is composed of the element carbon. Imagine taking a small piece of carbon and grinding it until it is a fine dust. Each speck of carbon would still have all of the physical and chemical properties of carbon. Now imagine that you could somehow keep dividing the speck of ca ...
... The graphite in your pencil is composed of the element carbon. Imagine taking a small piece of carbon and grinding it until it is a fine dust. Each speck of carbon would still have all of the physical and chemical properties of carbon. Now imagine that you could somehow keep dividing the speck of ca ...
Characteristics of Solids
... matter (atomic mass) increased, characteristics tended to repeat themselves in a predictable pattern. This was called “Periodicity.” When elements were placed in a table , those with similar properties were placed in a column, it produced vertical “Families.” ...
... matter (atomic mass) increased, characteristics tended to repeat themselves in a predictable pattern. This was called “Periodicity.” When elements were placed in a table , those with similar properties were placed in a column, it produced vertical “Families.” ...
Atomic Structure Powerpoint
... Number of protons in the nucleus of an atom This number is found on the Periodic Table Atomic Number identifies an element Always a positive number (b/c it is a counting ...
... Number of protons in the nucleus of an atom This number is found on the Periodic Table Atomic Number identifies an element Always a positive number (b/c it is a counting ...
Mass Defect - Lamont High
... Isotopes Elements can have atoms that contain the same number of protons but have different masses. The difference is due to the different number of neutrons in the nucleus. These are called isotopes. One example is carbon. It has three main isotopes: carbon-12, -13, and -14 ...
... Isotopes Elements can have atoms that contain the same number of protons but have different masses. The difference is due to the different number of neutrons in the nucleus. These are called isotopes. One example is carbon. It has three main isotopes: carbon-12, -13, and -14 ...
Chapter 4 Early Atomic Theory
... Average Atomic Mass How heavy is an atom of oxygen? There are different kinds of oxygen atoms, so the best way to represent the oxygen would be by using the average atomic mass. The average atomic mass is based on abundance of each isotope in nature. We do not use grams for mass because the numbers ...
... Average Atomic Mass How heavy is an atom of oxygen? There are different kinds of oxygen atoms, so the best way to represent the oxygen would be by using the average atomic mass. The average atomic mass is based on abundance of each isotope in nature. We do not use grams for mass because the numbers ...
The Chemistry of Life Chapter 2
... If they have to choose, atoms would rather be stable than neutral. ...
... If they have to choose, atoms would rather be stable than neutral. ...
Module1 for YIC CHEM
... The arrangements of elements in the increasing order of their atomic number with elements having similar properties placed in the same vertical columns (called groups) is known as periodic table. There are 18 groups (vertical columns) and 7 periods (horizontal rows) Elements belonging to the same gr ...
... The arrangements of elements in the increasing order of their atomic number with elements having similar properties placed in the same vertical columns (called groups) is known as periodic table. There are 18 groups (vertical columns) and 7 periods (horizontal rows) Elements belonging to the same gr ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.