CHE311 HEAT TRANSFER 2016-17 Fall Semester Prof.Dr.Serpil
... is 40 cm thick. Calculate the heat-transfer rate through the wall. 5. A copper sphere 4.0 cm in diameter is maintained at 70◦C and submerged in a large earth region where k =1.3 W/m ◦C. The temperature at a large distance from the sphere is 12◦C. Calculate the heat lost by the sphere. 6. Two long, e ...
... is 40 cm thick. Calculate the heat-transfer rate through the wall. 5. A copper sphere 4.0 cm in diameter is maintained at 70◦C and submerged in a large earth region where k =1.3 W/m ◦C. The temperature at a large distance from the sphere is 12◦C. Calculate the heat lost by the sphere. 6. Two long, e ...
Transfer of Thermal Energy worksheet - dubai
... If you have stood in front of a fireplace or near a campfire, you have felt the heat transfer known as radiation. The side of you nearest the fire warms, while your other side remains unaffected by the heat. Although you are surrounded by air, the air has nothing to do with this transfer of heat. He ...
... If you have stood in front of a fireplace or near a campfire, you have felt the heat transfer known as radiation. The side of you nearest the fire warms, while your other side remains unaffected by the heat. Although you are surrounded by air, the air has nothing to do with this transfer of heat. He ...
SPECIFIC HEAT
... CALORIMETRY & SPECIFIC HEAT THEORY Heat energy is defined as energy that flows from hot objects to cold objects. It can be measured in calories, kilocalories, or joules of energy. One calorie is defined as the amount of heat required to raise the temperature of 1 gram of water by 1 C. One calorie i ...
... CALORIMETRY & SPECIFIC HEAT THEORY Heat energy is defined as energy that flows from hot objects to cold objects. It can be measured in calories, kilocalories, or joules of energy. One calorie is defined as the amount of heat required to raise the temperature of 1 gram of water by 1 C. One calorie i ...
Specific Heat and Calculating Heat Absorbed - Varga
... It turns out that water has a much higher specific heat capacity than concrete does. The specific heat of concrete is 0.84 J/g°C, whereas the specific heat of water 4.184 J/g°C. If you have 1 kg of each substance at 0°C, which of them will take more energy to raise to a temperature of 50°C? ...
... It turns out that water has a much higher specific heat capacity than concrete does. The specific heat of concrete is 0.84 J/g°C, whereas the specific heat of water 4.184 J/g°C. If you have 1 kg of each substance at 0°C, which of them will take more energy to raise to a temperature of 50°C? ...
study Heat tr and density SG 2013 14
... your answer to one place beyond the decimal (to the tenths place). Be sure your units are correct! Show formula and math work for credit. ...
... your answer to one place beyond the decimal (to the tenths place). Be sure your units are correct! Show formula and math work for credit. ...
TW Series Key Features
... refrigerant. R-410A is a non-ozone depleting refrigerant since it does not contain any chlorine. R-410A is classified as an HFC (Hydrofluorocarbon) refrigerant as it only contains Hydrogen, Fluorine and Carbon. This refrigerant is the industry’s replacement for refrigerant R-22. Coaxial Heat Exchang ...
... refrigerant. R-410A is a non-ozone depleting refrigerant since it does not contain any chlorine. R-410A is classified as an HFC (Hydrofluorocarbon) refrigerant as it only contains Hydrogen, Fluorine and Carbon. This refrigerant is the industry’s replacement for refrigerant R-22. Coaxial Heat Exchang ...
(C, ° F ) u = internal energy (J/kg, Btu
... • We track this term in HVAC analysis • h = u + Pv or H=U+PV h = enthalpy (J/kg, Btu/lbm) P = Pressure (Pa, psi) v = specific volume (m3/kg, ft3/lbm) ...
... • We track this term in HVAC analysis • h = u + Pv or H=U+PV h = enthalpy (J/kg, Btu/lbm) P = Pressure (Pa, psi) v = specific volume (m3/kg, ft3/lbm) ...
Heat Transfer Oil
... Heat Transfer Oil is a highly refined and stable paraffinic oil designed to be used as a heat transfer medium and quenching oil. In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the he ...
... Heat Transfer Oil is a highly refined and stable paraffinic oil designed to be used as a heat transfer medium and quenching oil. In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the he ...
TEKNIK MESIN FAKULTAS TEKNOLOGI INDUSTRI UNIVERSITAS
... The transfer of heat is normally from a high temperature object to a lower temperature object. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. ...
... The transfer of heat is normally from a high temperature object to a lower temperature object. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. ...
E.ES.07.73 Fall 08
... The ocean has a time lag in which the effects of heating are felt on Earth. This lag in time occurs because the ocean has a high specific heat. The constant rising temperature of air takes significant time to affect the large masses of the oceans. Oceans are cooler than land in the summer, but warme ...
... The ocean has a time lag in which the effects of heating are felt on Earth. This lag in time occurs because the ocean has a high specific heat. The constant rising temperature of air takes significant time to affect the large masses of the oceans. Oceans are cooler than land in the summer, but warme ...
Weather Dynamics
... equally by climate change. • Low-lying and coastal areas face the risks associated with rising sea levels. • Scientists have also determined that warming will be greater in polar regions than nearer to the equator, and that continental interiors will experience greater warming than coastal areas. ...
... equally by climate change. • Low-lying and coastal areas face the risks associated with rising sea levels. • Scientists have also determined that warming will be greater in polar regions than nearer to the equator, and that continental interiors will experience greater warming than coastal areas. ...
Calculating heat stress index from routine weather
... • Climate change will increase temperatures in most places around the world in the coming decades. Temperatures in urban areas will go even higher due to the “heat island effect”. In order to measure the effect of climate change on worker productivity a heat stress index that incorporates temperatur ...
... • Climate change will increase temperatures in most places around the world in the coming decades. Temperatures in urban areas will go even higher due to the “heat island effect”. In order to measure the effect of climate change on worker productivity a heat stress index that incorporates temperatur ...
student powerpoint 3
... • Transfer of heat or cold via the movement of gas or liquid across an object, such as the body. • The heat molecules in the air are transferred over the skin. • The greater rate of movement the quicker the exchange can happen. • In cooler temps convection doesn’t allow the heat to leave the body. • ...
... • Transfer of heat or cold via the movement of gas or liquid across an object, such as the body. • The heat molecules in the air are transferred over the skin. • The greater rate of movement the quicker the exchange can happen. • In cooler temps convection doesn’t allow the heat to leave the body. • ...
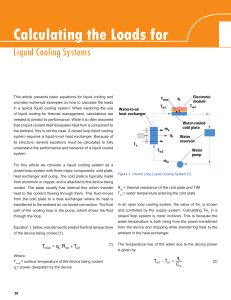
Calculating the Loads for Liquid cooling Systems
... provides numerical examples on how to calculate the loads in a typical liquid cooling system. When exploring the use of liquid cooling for thermal management, calculations are needed to predict its performance. While it is often assumed that a liquid coolant itself dissipates heat from a component t ...
... provides numerical examples on how to calculate the loads in a typical liquid cooling system. When exploring the use of liquid cooling for thermal management, calculations are needed to predict its performance. While it is often assumed that a liquid coolant itself dissipates heat from a component t ...
review sec 2 - Physics For Today
... pressure and it therefor expands. Because it expands, it does work. Since it does work it must loose energy. Therefor its temperature decreases. When air sinks, it is surrounded by air of higher pressure. The surrounding air compresses it, doing work to the sinking air. Since work is done to it, the ...
... pressure and it therefor expands. Because it expands, it does work. Since it does work it must loose energy. Therefor its temperature decreases. When air sinks, it is surrounded by air of higher pressure. The surrounding air compresses it, doing work to the sinking air. Since work is done to it, the ...
Chem 1010 Tutorials Tutorial 9A – Heat and Work Fall 2013
... In order to raise the temperature of a particular pond by 2.3 K, 5.2 x 1028 kJ of heat are required. a) What is the mass of the pond? (specific heat of water is 4.184 J·g–1·°C –1) b) What is the heat capacity of the pond? c) How much heat would be given off if the temperature of the pond decreased b ...
... In order to raise the temperature of a particular pond by 2.3 K, 5.2 x 1028 kJ of heat are required. a) What is the mass of the pond? (specific heat of water is 4.184 J·g–1·°C –1) b) What is the heat capacity of the pond? c) How much heat would be given off if the temperature of the pond decreased b ...
Specific and latent heat
... 4. What is meant by the specific heat capacity of a substance? 5. How much heat energy is needed to heat 4kg of aluminium by 80C? [Specific Heat Capacity of aluminium = 1200 J/(kg K)]. 6. If 48 000 J of heat energy are given off when a 2 kg block of metal cools by 120C, what is the specific heat cap ...
... 4. What is meant by the specific heat capacity of a substance? 5. How much heat energy is needed to heat 4kg of aluminium by 80C? [Specific Heat Capacity of aluminium = 1200 J/(kg K)]. 6. If 48 000 J of heat energy are given off when a 2 kg block of metal cools by 120C, what is the specific heat cap ...
Heat, Enthalpy, Temperature
... Careful: In chemistry, a glass of hot water does not HAVE heat. A glass of hot water has ENERGY which is given off in the form of heat. ...
... Careful: In chemistry, a glass of hot water does not HAVE heat. A glass of hot water has ENERGY which is given off in the form of heat. ...
Physical Property Notes
... How do we calculate specific heat in the lab? 1) Heat up a known mass of a metal in boiling water until the substance has reached the same temperature as the boiling water (100oC) 2) Transfer (very quickly) the hot metal to a water bath that contains a specific amount of water at a known temperatur ...
... How do we calculate specific heat in the lab? 1) Heat up a known mass of a metal in boiling water until the substance has reached the same temperature as the boiling water (100oC) 2) Transfer (very quickly) the hot metal to a water bath that contains a specific amount of water at a known temperatur ...
11-Heat Energy
... must be added to raise the temperature of an object. A large heat capacity means that a lot of heat must be added transferred to raise the temperature of the object by a given amount. A bigger object of the same material has a bigger heat capacity. ...
... must be added to raise the temperature of an object. A large heat capacity means that a lot of heat must be added transferred to raise the temperature of the object by a given amount. A bigger object of the same material has a bigger heat capacity. ...
DriTherm®: Brick Cavity Wall Insulation
... During the hot summer months, DriTherm Cavity slab will help to keep the inside of the house cool by restricting heat radiating in to the home from the outside brickwork. ...
... During the hot summer months, DriTherm Cavity slab will help to keep the inside of the house cool by restricting heat radiating in to the home from the outside brickwork. ...
Chemistry 2015-2016 Name: Calorimetry Practice Date: Per
... q means heat is released. + q means heat is absorbed. T is always = final temperature – initial temperature. If something is getting hotter (10° 30°) the T is 30 – 10 = + 20°. (heat is absorbed) If something is getting cooler (75° 25°) the T is 25 – 75 = 50°. (heat is released) ...
... q means heat is released. + q means heat is absorbed. T is always = final temperature – initial temperature. If something is getting hotter (10° 30°) the T is 30 – 10 = + 20°. (heat is absorbed) If something is getting cooler (75° 25°) the T is 25 – 75 = 50°. (heat is released) ...
Intercooler

An intercooler is any mechanical device used to cool a fluid, including liquids or gases, between stages of a multi-stage heating process, typically a heat exchanger that removes waste heat in a gas compressor. They are used in many applications, including air compressors, air conditioners, refrigerators, and gas turbines, and are widely known in automotive use as an air-to-air or air-to-liquid cooler for forced induction (turbocharged or supercharged) internal combustion engines to improve their volumetric efficiency by increasing intake air charge density through nearly isobaric (constant pressure) cooling.