Heat illness
... During normal work activities, especially, during summer months, workers may be required to work in hot environments. When the body is unable to cool itself by sweating, several heat-induced illnesses can occur, and can result in death. ...
... During normal work activities, especially, during summer months, workers may be required to work in hot environments. When the body is unable to cool itself by sweating, several heat-induced illnesses can occur, and can result in death. ...
2016 Q7 - Loreto Balbriggan
... As part of his presentation, Joule proposed that the temperature of the water at the bottom of the Niagara Falls would be 0.12 °C greater than that at the top, due to gravitational potential energy being converted into heat energy. Calculate the height of the Niagara Falls. In reality the increase i ...
... As part of his presentation, Joule proposed that the temperature of the water at the bottom of the Niagara Falls would be 0.12 °C greater than that at the top, due to gravitational potential energy being converted into heat energy. Calculate the height of the Niagara Falls. In reality the increase i ...
Chapter 11 1. While checking the temperature of an IC. chip the
... flowed through that one side? ...
... flowed through that one side? ...
POWERPOINT SCIENCE
... The measure of the average kinetic energy of the individual particles in an object. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion even if the object they make up isn’t moving at all. Energy of motion is called kinetic energy, so all particle ...
... The measure of the average kinetic energy of the individual particles in an object. Matter is made up of tiny particles called atoms and molecules. These particles are always in motion even if the object they make up isn’t moving at all. Energy of motion is called kinetic energy, so all particle ...
saving with heat pumps
... your zone—zones 1 and 2 (map below) are generally warm enough to allow heat pumps to operate efficiently. Generally, the yearly average ambient temperature should be 19 degrees Celsius or higher how many STCs—the number of STCs (or Small-scale Technology Certificates) for each system also depends on ...
... your zone—zones 1 and 2 (map below) are generally warm enough to allow heat pumps to operate efficiently. Generally, the yearly average ambient temperature should be 19 degrees Celsius or higher how many STCs—the number of STCs (or Small-scale Technology Certificates) for each system also depends on ...
Chapter 12
... • So the internal energy could be transferred as mechanical energy in the form of work. • Recall that work required some displacement to exist, we also need that fluid to create a displacement. • So work can only be done when there is a change in volume. – The pressure should remain constant. – If n ...
... • So the internal energy could be transferred as mechanical energy in the form of work. • Recall that work required some displacement to exist, we also need that fluid to create a displacement. • So work can only be done when there is a change in volume. – The pressure should remain constant. – If n ...
Thermodynamics-d2
... surroundings due to organized motion in the surroundings. (rubbing a block of wood vigorously, stir a glass of water, allow a gas to expand against an external pressure.) ...
... surroundings due to organized motion in the surroundings. (rubbing a block of wood vigorously, stir a glass of water, allow a gas to expand against an external pressure.) ...
Types of Heat Related Illnesses
... vegetables count as part of your water intake. If you are thirsty you are already dehydrated or on your way to dehydration. ...
... vegetables count as part of your water intake. If you are thirsty you are already dehydrated or on your way to dehydration. ...
CONVECTION HEAT TRANSFER Figure
... forced flow induced by external agency e.g. pump eg. forced-draught air cooler, evaporators ...
... forced flow induced by external agency e.g. pump eg. forced-draught air cooler, evaporators ...
Specific Heat and Calorimeters
... goes from 23.0 OC to 30 OC. Find the heat transfer (Q) for the water. Specific heat (c) water = 4.184 J/g •OC ...
... goes from 23.0 OC to 30 OC. Find the heat transfer (Q) for the water. Specific heat (c) water = 4.184 J/g •OC ...
www.koldkatcher.com Industrial Heat Trace Systems HEATED FUEL GAS THIEF HATCH HEATERS
... Recommended when installation is not permanent as with oil wells with short life cycle. Hose can be removed readily, cuts down on working pressure of 100 psi @180F installation costs, easily installed at Burst pressure of 600 psi @ 180F any temperature, flame and freeze 25 year warranty resistant, k ...
... Recommended when installation is not permanent as with oil wells with short life cycle. Hose can be removed readily, cuts down on working pressure of 100 psi @180F installation costs, easily installed at Burst pressure of 600 psi @ 180F any temperature, flame and freeze 25 year warranty resistant, k ...
Heat, Temperature and Atmospheric Circulations
... • In equilibrium temperature is constant, though different parts may be different temperatures ...
... • In equilibrium temperature is constant, though different parts may be different temperatures ...
WORKSHOP 2: Dimensional Analysis and
... 7. A rubber stopper weighing 65.4 g is immersed into a graduated cylinder filled with 30.0 mL of liquid. The liquid level then rises to 48.8 mL. Calculate the density of the stopper. 3.48 g/mL 8. If the density of a liquid is known to be 0.785 g/mL, calculate the mass of the liquid if its volume is ...
... 7. A rubber stopper weighing 65.4 g is immersed into a graduated cylinder filled with 30.0 mL of liquid. The liquid level then rises to 48.8 mL. Calculate the density of the stopper. 3.48 g/mL 8. If the density of a liquid is known to be 0.785 g/mL, calculate the mass of the liquid if its volume is ...
Heat exchanger design for hot air ericsson
... used gas combustion engines, as fuel is used natural gas. Into the engine is transferred fuel, through combustion we are gaining mechanical work on the output shaft and the heat energy is transferred through cooling system to the cooling heat exchanger. Additionally we may collect thermal energy fro ...
... used gas combustion engines, as fuel is used natural gas. Into the engine is transferred fuel, through combustion we are gaining mechanical work on the output shaft and the heat energy is transferred through cooling system to the cooling heat exchanger. Additionally we may collect thermal energy fro ...
GAIN AN OVERVIEW OF THE EXPERIMENT
... flight metabolic rate and wingbeat frequency. This result suggests the hypothesis that honeybees may be able to vary their metabolic rate by changing flight muscle performance, and in this way achieve thermoregulation. However, the observed correlation might have other explanations. The air temperat ...
... flight metabolic rate and wingbeat frequency. This result suggests the hypothesis that honeybees may be able to vary their metabolic rate by changing flight muscle performance, and in this way achieve thermoregulation. However, the observed correlation might have other explanations. The air temperat ...
thermodynamics
... food off, how much equivalent mechanical work would you have to do (in Joules please)? (Note: 4.186 J = 1 cal. The definition of a “cal” is the amount of heat required to raise 1 g of water 1 degree celsius–i.e. the specific heat. Memorize this number.) 1500 Cal A (1000 cal/1 Cal) A (4.186 J/1cal) = ...
... food off, how much equivalent mechanical work would you have to do (in Joules please)? (Note: 4.186 J = 1 cal. The definition of a “cal” is the amount of heat required to raise 1 g of water 1 degree celsius–i.e. the specific heat. Memorize this number.) 1500 Cal A (1000 cal/1 Cal) A (4.186 J/1cal) = ...
Quiz-1_MA
... The volume of an iron cube, 5 cm on edge, will increase by what amount if it is heated from 10°C to 60°C? A) ...
... The volume of an iron cube, 5 cm on edge, will increase by what amount if it is heated from 10°C to 60°C? A) ...
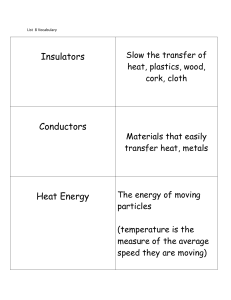
List 8 Vocabulary Cards - Endeavor Charter School
... Mixing, but the item is still the same substance, reversible ...
... Mixing, but the item is still the same substance, reversible ...
Heat Transfer: Conduction, Convection and Latent Heat In addition
... That 51% absorbed by the ground then gets distributed throughout the atmosphere...... ...
... That 51% absorbed by the ground then gets distributed throughout the atmosphere...... ...
Development of a Design Tool for Hot-Dry-Rock Fracture
... The Hot-Dry-Rock (HDR) technology has great potential for large-scale conversion of geothermal energy into electric energy. Pressurized water is used to fracture hot rock at depth of about 3-5 km in order to create an engineered underground heat exchanger. For producing geothermal heat, cold water i ...
... The Hot-Dry-Rock (HDR) technology has great potential for large-scale conversion of geothermal energy into electric energy. Pressurized water is used to fracture hot rock at depth of about 3-5 km in order to create an engineered underground heat exchanger. For producing geothermal heat, cold water i ...
Thermal Expansion and Temperature Scales
... 1. Why do your hands feel warmer when you hold a cup of hot chocolate? 2. While grilling hamburgers, the meat is placed directly over the coals instead of to the side of the coals to increase the heat transfer by _______ 3. The transfer of energy that does not require any matter is _____________. 4. ...
... 1. Why do your hands feel warmer when you hold a cup of hot chocolate? 2. While grilling hamburgers, the meat is placed directly over the coals instead of to the side of the coals to increase the heat transfer by _______ 3. The transfer of energy that does not require any matter is _____________. 4. ...
Specific Heat Capacity - Tasker Milward
... Specific Heat Capacity • The specific heat capacity is the amount of energy required to increase the temperature of 1kg of a substance by 1˚C • We will calculate the specific heat capacity of water by heating it with an electrical heater and measuring the energy required for a fixed temperature ris ...
... Specific Heat Capacity • The specific heat capacity is the amount of energy required to increase the temperature of 1kg of a substance by 1˚C • We will calculate the specific heat capacity of water by heating it with an electrical heater and measuring the energy required for a fixed temperature ris ...
Thermodynamics
... Heat is a form of energy so we can always use Joules. More common in thermodynamics is the calorie: By definition 1 calorie is the amount of heat required to change the temperature of 1 gram of water 1°C. ...
... Heat is a form of energy so we can always use Joules. More common in thermodynamics is the calorie: By definition 1 calorie is the amount of heat required to change the temperature of 1 gram of water 1°C. ...
2nd law - WordPress.com
... It is impossible to construct a device which operating in a cycle will produce no effect other than transfer of heat from a cooler to a hotter body. Kelvin-plank statement ...
... It is impossible to construct a device which operating in a cycle will produce no effect other than transfer of heat from a cooler to a hotter body. Kelvin-plank statement ...
Heat Transfer - Madison County Schools
... liquid or gas (or plasma). The particles of a fluid take heat with them as they move. If you heat the air in one room, the air will heat the next room as the air flows from one room to the next. This is heating by convection. ...
... liquid or gas (or plasma). The particles of a fluid take heat with them as they move. If you heat the air in one room, the air will heat the next room as the air flows from one room to the next. This is heating by convection. ...
Intercooler

An intercooler is any mechanical device used to cool a fluid, including liquids or gases, between stages of a multi-stage heating process, typically a heat exchanger that removes waste heat in a gas compressor. They are used in many applications, including air compressors, air conditioners, refrigerators, and gas turbines, and are widely known in automotive use as an air-to-air or air-to-liquid cooler for forced induction (turbocharged or supercharged) internal combustion engines to improve their volumetric efficiency by increasing intake air charge density through nearly isobaric (constant pressure) cooling.