specific heat
... How much energy would be needed to heat 450 g of copper metal from 25.0 ºC to 75.0 ºC? The specific heat of copper at 25.0 ºC is 0.385 J/g ºC. ...
... How much energy would be needed to heat 450 g of copper metal from 25.0 ºC to 75.0 ºC? The specific heat of copper at 25.0 ºC is 0.385 J/g ºC. ...
Thermodynamics!!!
... COLD, only a lack of heat Temperature is the measurement of the average kinetic energy of the molecules of a substance Heat always moves from “warm to cold” meaning from something with a higher temperature to ...
... COLD, only a lack of heat Temperature is the measurement of the average kinetic energy of the molecules of a substance Heat always moves from “warm to cold” meaning from something with a higher temperature to ...
Test Review-Atmosphere Intro
... Test 2: Intro/Properties of Earth’s Atmosphere The following is a list of topics to help guide you in your studies. This is not to be used as your only source of studying!!! Topics on the exam may include but are not limited to the following: 1. ESRT Temperature & Pressure a. Reading both charts, co ...
... Test 2: Intro/Properties of Earth’s Atmosphere The following is a list of topics to help guide you in your studies. This is not to be used as your only source of studying!!! Topics on the exam may include but are not limited to the following: 1. ESRT Temperature & Pressure a. Reading both charts, co ...
Heat Recovery for Commercial Buildings
... One of the simplest forms of equipment used for heat recovery. Efficiencies range between 50 & 70%. Conduction, which is a mode of energy transfer between two ...
... One of the simplest forms of equipment used for heat recovery. Efficiencies range between 50 & 70%. Conduction, which is a mode of energy transfer between two ...
Name____________________________
... Convection: Transfer of heat within a liquid or gas. Conduction: Transfer of heat through matter by direct contact. Thermal Radiation: The energy radiated by solids, liquids, and gases in the form of electromagnetic waves as a result of their temperature. Deformation: Alteration of shape, as by pres ...
... Convection: Transfer of heat within a liquid or gas. Conduction: Transfer of heat through matter by direct contact. Thermal Radiation: The energy radiated by solids, liquids, and gases in the form of electromagnetic waves as a result of their temperature. Deformation: Alteration of shape, as by pres ...
Ch.19 (section 1 only)
... Second Law of Thermodynamics • Heat flows spontaneously from a substance at a higher temperature to a substance at a lower temperature. • The reverse situation does not occur spontaneously. • Ex. Hot drink cools but does not reheat ...
... Second Law of Thermodynamics • Heat flows spontaneously from a substance at a higher temperature to a substance at a lower temperature. • The reverse situation does not occur spontaneously. • Ex. Hot drink cools but does not reheat ...
INTERNAL COMBUSTION ENGINE - Area10FFA
... back to the history of the combustion engine. James Watt realized that a steam engine produced more power than anyone human. He needed a reference point to compare the power of his new steam engine. He selected a horse and determined that a horse could move/lift 33,000 lbs on a linear plane, 1 foot ...
... back to the history of the combustion engine. James Watt realized that a steam engine produced more power than anyone human. He needed a reference point to compare the power of his new steam engine. He selected a horse and determined that a horse could move/lift 33,000 lbs on a linear plane, 1 foot ...
Heat Transfer Comparison in Coaxial Tube in Tube Heat Exchanger
... are currently used in R&AC systems, in accordance with the Montreal Protocol, actual is exchange of those by new, ecologically acceptable, HFC refrigerants. Therefore system performance analyses was made where the single component refrigerant R22 was replaced with zeotropic mixture R407C. In the sys ...
... are currently used in R&AC systems, in accordance with the Montreal Protocol, actual is exchange of those by new, ecologically acceptable, HFC refrigerants. Therefore system performance analyses was made where the single component refrigerant R22 was replaced with zeotropic mixture R407C. In the sys ...
Chapters 1 and 2
... Temperature is the thing that’s the same for two objects, after they’ve been in contact long enough. Long enough so that the two objects are in thermal equilibrium. Time required to reach thermal equilibrium is the relaxation time. Temperature is usually measured in K, C or F and cannot be expres ...
... Temperature is the thing that’s the same for two objects, after they’ve been in contact long enough. Long enough so that the two objects are in thermal equilibrium. Time required to reach thermal equilibrium is the relaxation time. Temperature is usually measured in K, C or F and cannot be expres ...
Notes - hrsbstaff.ednet.ns.ca
... Example 3. Solving for temperature (∆T then final temperature) ...
... Example 3. Solving for temperature (∆T then final temperature) ...
science grade 7 blizzard bag assignment
... substance to another substance that is touching is called conduction. Conduction works well in some solids, but not as well in fluids (liquids and gases). In convection, heat is transferred by the movement of currents within a fluid. In fluids, molecules can move from place to place and take their h ...
... substance to another substance that is touching is called conduction. Conduction works well in some solids, but not as well in fluids (liquids and gases). In convection, heat is transferred by the movement of currents within a fluid. In fluids, molecules can move from place to place and take their h ...
2 Pieces - cloudfront.net
... Insulators serve to (increase, decrease or not change) the transfer of heat energy. ...
... Insulators serve to (increase, decrease or not change) the transfer of heat energy. ...
Exercises – Chapter 8
... E.18 The hotter the water, the more thermal energy can be harnessed and the greater the temperature difference between the hot water and the colder surrounding, the more efficient the hurricane is at converting that thermal energy into mechanical work. 19. On a clear sunny day, the ground is heated ...
... E.18 The hotter the water, the more thermal energy can be harnessed and the greater the temperature difference between the hot water and the colder surrounding, the more efficient the hurricane is at converting that thermal energy into mechanical work. 19. On a clear sunny day, the ground is heated ...
Chapter 3: Air Temperature
... Air Temperature and Human Comfort • Human body stabilizes its T (i.e., prevents its T decrease) primarily by converting food into heat (metabolism) • The stronger the wind, the faster the body’s heat loss • High winds in below-freezing air can remove heat from exposed skin so quickly that the skin ...
... Air Temperature and Human Comfort • Human body stabilizes its T (i.e., prevents its T decrease) primarily by converting food into heat (metabolism) • The stronger the wind, the faster the body’s heat loss • High winds in below-freezing air can remove heat from exposed skin so quickly that the skin ...
NOTES-Chapter 12
... •Temperature and heat are different. Temperature is a measure of the average kinetic energy of the molecules of the substance. ...
... •Temperature and heat are different. Temperature is a measure of the average kinetic energy of the molecules of the substance. ...
Teacher:
... transfer during chemical reactions and changes of state is called thermochemistry. One of the units used to measure heat flow is the calories defined as the amount of heat needed to raise 1 g of water 1oC. The SI unit of heat and energy is joule, which is equal to 0.2390 cal. The specific heat capac ...
... transfer during chemical reactions and changes of state is called thermochemistry. One of the units used to measure heat flow is the calories defined as the amount of heat needed to raise 1 g of water 1oC. The SI unit of heat and energy is joule, which is equal to 0.2390 cal. The specific heat capac ...
03. Energy and Conservation Laws
... — the physical properties (such as volume) of each object may change right after it was placed in contact with other ones. — as the time goes long enough, the physical properties of the objects are no longer changing. These objects are said to be in thermal equilibrium. We said “They have the same t ...
... — the physical properties (such as volume) of each object may change right after it was placed in contact with other ones. — as the time goes long enough, the physical properties of the objects are no longer changing. These objects are said to be in thermal equilibrium. We said “They have the same t ...
Cases – Chapter 8 1. A solar panel is an electronic device that
... c. When the shift was over, the doors to the caisson were opened and the air was allowed to rush out. That outgoing air was ice cold. The air inside the caisson was also so cold that sometimes it would begin to snow. What caused this temperature drop? d. When during this cycle of pressurizing and de ...
... c. When the shift was over, the doors to the caisson were opened and the air was allowed to rush out. That outgoing air was ice cold. The air inside the caisson was also so cold that sometimes it would begin to snow. What caused this temperature drop? d. When during this cycle of pressurizing and de ...
Skills Worksheet
... basement. The hot gases from the combustion of wood or coal rose through the ducts and provided heat for the building. After the fall of the Roman Empire, these heating pipes disappeared. People used open fires and fireplaces. One problem with fireplaces is that 80 percent of the heat escapes up the ...
... basement. The hot gases from the combustion of wood or coal rose through the ducts and provided heat for the building. After the fall of the Roman Empire, these heating pipes disappeared. People used open fires and fireplaces. One problem with fireplaces is that 80 percent of the heat escapes up the ...
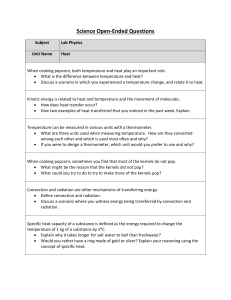
Physics-Heat OEQs
... Specific heat capacity of a substance is defined as the energy required to change the temperature of 1 kg of a substance by 1⁰C. Explain why it takes longer for salt water to boil than freshwater? Would you rather have a ring made of gold or silver? Explain your reasoning using the concept of sp ...
... Specific heat capacity of a substance is defined as the energy required to change the temperature of 1 kg of a substance by 1⁰C. Explain why it takes longer for salt water to boil than freshwater? Would you rather have a ring made of gold or silver? Explain your reasoning using the concept of sp ...
Calorimetry: Heat of Fusion of Ice Procedure In a 250 mL beaker
... Immediately add 2-3 ice cubes. Stir the mixture carefully. The cup should contain ice at all times. If the last of the ice is about to melt, add another ice cube. Monitor the temperature of the mixture as you stir. Continue stirring until the temperature no longer drops. Record this final temperatur ...
... Immediately add 2-3 ice cubes. Stir the mixture carefully. The cup should contain ice at all times. If the last of the ice is about to melt, add another ice cube. Monitor the temperature of the mixture as you stir. Continue stirring until the temperature no longer drops. Record this final temperatur ...
Thermal Energy
... object Of higher temperature to an object of lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
... object Of higher temperature to an object of lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
Intercooler

An intercooler is any mechanical device used to cool a fluid, including liquids or gases, between stages of a multi-stage heating process, typically a heat exchanger that removes waste heat in a gas compressor. They are used in many applications, including air compressors, air conditioners, refrigerators, and gas turbines, and are widely known in automotive use as an air-to-air or air-to-liquid cooler for forced induction (turbocharged or supercharged) internal combustion engines to improve their volumetric efficiency by increasing intake air charge density through nearly isobaric (constant pressure) cooling.