* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download (C, ° F ) u = internal energy (J/kg, Btu
Survey
Document related concepts
Indoor air quality wikipedia , lookup
Fan (machine) wikipedia , lookup
Radiator (engine cooling) wikipedia , lookup
Heat equation wikipedia , lookup
Solar water heating wikipedia , lookup
Heat exchanger wikipedia , lookup
R-value (insulation) wikipedia , lookup
Dynamic insulation wikipedia , lookup
Underfloor heating wikipedia , lookup
Thermal conduction wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Cogeneration wikipedia , lookup
Intercooler wikipedia , lookup
Vapor-compression refrigeration wikipedia , lookup
Hyperthermia wikipedia , lookup
Transcript
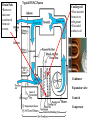
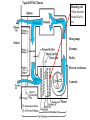
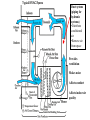
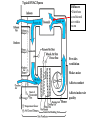
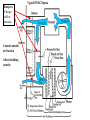
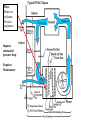
Objectives • Review thermodynamics - Solve thermodynamic problems and use properties in equations, tables and diagrams Systems: Heating • Make heat (furnace, boiler, solar, etc.) • Distribute heat within building (pipes, ducts, fans, pumps) • Exchange heat with air (coils, strip heat, radiators, convectors, diffusers) • Controls (thermostat, valves, dampers) Systems: Cooling • Absorb heat from building (evaporator or chilled water coil) • Reject heat to outside (condenser) • Refrigeration cycle components (expansion valve, compressor, concentrator, absorber, refrigerant) • Distribute cooling within building (pipes, ducts, fans, pumps) • Exchange cooling with air (coils, radiant panels, convectors, diffusers) • Controls (thermostat, valves, dampers, reheat) Systems: Ventilation • Fresh air intake (dampers, economizer, heat exchangers, primary treatment) • Air exhaust (dampers, heat exchangers) • Distribute fresh air within building (ducts, fans) • Air treatment (filters, etc.) • Controls (thermostat, CO2 and other occupancy sensors, humidistats, valves, dampers) Systems: Other • Auxiliary systems (i.e. venting of combustion gasses) • Condensate drainage/return • Dehumidification (desiccant, cooling coil) • Humidification (steam, ultrasonic humidifier) • Energy management systems Drain Pain •Removes moisture condensed from air stream Cooling coil •Heat transfer from air to refrigerant •Extended surface coil Condenser Expansion valve Controls Compressor Heating coil •Heat transfer from fluid to air Heat pump Furnace Boiler Electric resistance Controls Blower •Overcome pressure drop of system Adds heat to air stream Makes noise Potential hazard Performs differently at different conditions (air flow and pressure drop) Duct system (piping for hydronic systems) •Distribute conditioned air •Remove air from space Provides ventilation Makes noise Affects comfort Affects indoor air quality Diffusers •Distribute conditioned air within room Provides ventilation Makes noise Affects comfort Affects indoor air quality Dampers •Change airflow amounts Controls outside air fraction Affects building security Filter •Removes pollutants •Protects equipment Imposes substantial pressure drop Requires Maintenance Controls •Makes everything work Temperature Pressure (drop) Air velocity Volumetric flow Relative humidity Enthalpy Electrical Current Electrical cost Fault detection Review • Basic units • Thermodynamics processes in HVAC systems Heat Units • Heat = energy transferred because of a temperature difference • Btu = energy required to raise 1 lbm of water 1 °F • kJ • Specific heat (heat per unit mass) • Btu/(lbm∙°F), kJ/(kg∙°C) • For gasses, two relevant quantities cv and cp • Basic equation (2.10) Q = mcΔt Q = heat transfer (Btu, kJ) m = mass (kg, lbm) c = specific heat Δt = temperature difference Sensible vs. latent heat • Sensible heat Q = mcΔt • Latent heat is associate with change of phase at constant temperature • • • • Latent heat of vaporization, hfg Latent heat of fusion, hfi hfg for water (100 °C, 1 atm) = 1220 Btu/lbm hfi for ice (0 °C, 1 atm) = 144 Btu/lbm Work, Energy, and Power • Work is energy transferred from system to surroundings when a force acts through a distance • ft∙lbf or N∙m (note units of energy) • Power is the time rate of work performance • Btu/hr or W • Unit conversions in Handouts • 1 ton = 12,000 Btu/hr (HVAC specific) Where does 1 ton come from? • • • • • • 1 ton = 2000 lbm Energy released when 2000 lbm of ice melts = 2000 lbm × 144 BTU/lbm = 288 kBTU Process is assumed to take 1 day (24 hours) 1 ton of air conditioning = 12 kBTU/hr Note that it is a unit of power (energy/time) Thermodynamic Laws • First law? • Second law? • Implications for HVAC • Need a refrigeration machine (and external energy) to make energy flow from cold to hot • Literature for Thermodynamics Review: • Fundamentals of Thermal-Fluid Sciences -Yunus A. Cengel, Robert H. Turner, Yunus Cengel, Robert Turner • or any thermodynamics book Internal Energy and Enthalpy • 1st law says energy is neither created or destroyed • So, we must be able to store energy • Internal energy (u) is all energy stored • Molecular vibration, rotation, etc. • Formal definition in statistical thermodynamics • Enthalpy • Total energy • We track this term in HVAC analysis • h = u + Pv or H=U+PV h = enthalpy (J/kg, Btu/lbm) P = Pressure (Pa, psi) v = specific volume (m3/kg, ft3/lbm) Second law In any cyclic process the entropy will either increase or remain the same. Entropy • Not directly measurable • Mathematical construct dQ dS T or Q dS T S = entropy (J/K, BTU/°R) Q = heat (J, BTU) T = absolute temperature (K, °R) • Note difference between s and S • Entropy can be used as a condition for equilibrium T-s diagrams • dH = TdS + VdP (general property equation) • Area under T-s curve is change in specific energy – under what condition? T-s diagram h-s diagram p-h diagram Ideal gas law • Pv = RT or PV = nRT • R is a constant for a given fluid • For perfect gasses • Δu = cvΔt • Δh = cpΔt • cp - cv= R 1545 ft lbf 8.314 kJ R M lbm R M kg K M = molecular weight (g/mol, lbm/mol) P = pressure (Pa, psi) V = volume (m3, ft3) v = specific volume (m3/kg, ft3/lbm) T = absolute temperature (K, °R) t = temperature (C, °F) u = internal energy (J/kg, Btu, lbm) h = enthalpy (J/kg, Btu/lbm) n = number of moles (mol) Mixtures of Perfect Gasses • • • • • m = mx my Px V = mx R ∙T V = Vx Vy Py V = my R ∙T T = Tx Ty What is ideal gas law for mixture? P = Px Py Assume air is an ideal gas x y • -70 °C to 80 °C (-100 °F to 180 °F) m = mass (g, lbm) P = pressure (Pa, psi) V = volume (m3, ft3) R = material specific gas constant T = absolute temperature (K, °R) Mass-Weighted Averages • Quality, x, is mg/(mf + mg) • Vapor mass fraction • φ= v or h or s in expressions below • φ = φf + x φfg s = entropy (J/K/kg, BTU/°R/lbm) m = mass (g, lbm) • φ = (1- x) φf + x φg h = enthalpy (J/kg, Btu/lbm) v = specific volume (m3/kg) Subscripts f and g refer to saturated liquid and vapor states and fg is the difference between the two Properties of water • Water, water vapor (steam), ice • Properties of water and steam (pg 675 – 685) • Alternative - ASHRAE Fundamentals ch. 6