12.748 Lecture 2 Cosmic Abundances, Nucleosynthesis and
... (vertical axis) plotted against its apparent, surface temperature. After a few tens of thousands of years, the star has become so hot that its thermal energy exceeds the barrier between deuterium nuclei (deuterium is the heavier isotope of hydrogen, whose nucleus consists of one proton and one neutr ...
... (vertical axis) plotted against its apparent, surface temperature. After a few tens of thousands of years, the star has become so hot that its thermal energy exceeds the barrier between deuterium nuclei (deuterium is the heavier isotope of hydrogen, whose nucleus consists of one proton and one neutr ...
Age, EvoluFon, and Size of the Cosmos
... • Protons and neutrons are able to bind together to form nuclei since their binding energy is now greater than the cosmic background radia+on energy, so the background of light (photons) can’t break th ...
... • Protons and neutrons are able to bind together to form nuclei since their binding energy is now greater than the cosmic background radia+on energy, so the background of light (photons) can’t break th ...
15 Billion
... of Moon. Oldest fossils are about 3.8 by old. f. Mathematical models predict that stars the size of the Sun will undergo nuclear fusion in their core. g. All galaxies are red-shifting, i.e., the universe is expanding. Cosmic background radiation, a remnant of the big bang, is observed. h. Hubble spa ...
... of Moon. Oldest fossils are about 3.8 by old. f. Mathematical models predict that stars the size of the Sun will undergo nuclear fusion in their core. g. All galaxies are red-shifting, i.e., the universe is expanding. Cosmic background radiation, a remnant of the big bang, is observed. h. Hubble spa ...
A SUMMARY OF SELF
... 3. The lightest isotopes of many heavy elements are very rare compared to the heavier isotopes, How can this difference be explained? 4. Discuss the time scale for the s-process? What life-time must a nucleus have in order to have time to capture a neutron before it β-decays? The r-process 1. How ma ...
... 3. The lightest isotopes of many heavy elements are very rare compared to the heavier isotopes, How can this difference be explained? 4. Discuss the time scale for the s-process? What life-time must a nucleus have in order to have time to capture a neutron before it β-decays? The r-process 1. How ma ...
(the factor f star in the Drake equation. Recall it
... FIRST TWO elements (hydrogen and helium) is complex enough to result in anything at all lifelike. (Some might even dispute this conclusion.) With that assumption, and some information about how stars form, we can derive an estimate of fstar ...
... FIRST TWO elements (hydrogen and helium) is complex enough to result in anything at all lifelike. (Some might even dispute this conclusion.) With that assumption, and some information about how stars form, we can derive an estimate of fstar ...
Stellar Evolution Reading Questions Integrated Science 2 Name
... 3. Most stars go through the same three stages before they die. Describe each stage below. Include how long it lasts, what the main events are, and what causes it to move to the next stage. ...
... 3. Most stars go through the same three stages before they die. Describe each stage below. Include how long it lasts, what the main events are, and what causes it to move to the next stage. ...
Elements from Stardust
... Stars the size of the sun do not contain enough energy to produce heavier elements than oxygen. ...
... Stars the size of the sun do not contain enough energy to produce heavier elements than oxygen. ...
PPT
... involving iron do not release energy • Iron-56 has lowest mass per nuclear particle • Highest “binding energy” of all the elements ...
... involving iron do not release energy • Iron-56 has lowest mass per nuclear particle • Highest “binding energy” of all the elements ...
112501. r-process beam neutron
... QuickTime™ and a Video decompressor are needed to see this picture. ...
... QuickTime™ and a Video decompressor are needed to see this picture. ...
Slides from Lecture09
... little energy is released through chemical burning processes. – To generate the Sun’s luminosity via chemical burning, the entire Sun would be consumed in about 10,000 years! ...
... little energy is released through chemical burning processes. – To generate the Sun’s luminosity via chemical burning, the entire Sun would be consumed in about 10,000 years! ...
More detailed notes
... between helium and carbon. These are noticeably uncommon, as can be seen in the first plot on page 2, but they do exist, so they must be formed somehow. They are definitely not made by fusion, since (as shown in the second plot on page 2) they are actually less tightly bound than 4He. It is believed ...
... between helium and carbon. These are noticeably uncommon, as can be seen in the first plot on page 2, but they do exist, so they must be formed somehow. They are definitely not made by fusion, since (as shown in the second plot on page 2) they are actually less tightly bound than 4He. It is believed ...
ASTRONOMY 220C ADVANCED STAGES OF
... advanced stages of the evolution of massive stars; c) nucleosynthesis; d) supernovae of all types and sorts, and e) other forms of explosive astrophysical transients (novae, x-ray bursts, gamma-ray bursts). Our study of supernovae will be extensive and will include not only the mechanisms and evolut ...
... advanced stages of the evolution of massive stars; c) nucleosynthesis; d) supernovae of all types and sorts, and e) other forms of explosive astrophysical transients (novae, x-ray bursts, gamma-ray bursts). Our study of supernovae will be extensive and will include not only the mechanisms and evolut ...
Electromagnetic Radiation from the Sun
... atomic number elements. This is the case because of the Big Bang, when temperatures were so high that only energy could exist. As the universe expanded, it cooled and some of the energy converted into matter in the form of electrons, protons and neutrons. As the universe continued to expand and cool ...
... atomic number elements. This is the case because of the Big Bang, when temperatures were so high that only energy could exist. As the universe expanded, it cooled and some of the energy converted into matter in the form of electrons, protons and neutrons. As the universe continued to expand and cool ...
Fingerprints in Starlight: Spectroscopy of Stars Inquiry Questions
... is the case because of the Big Bang, when temperatures were so high that only energy could exist. As the universe expanded, it cooled and some of the energy converted into matter in the form of electrons, protons and neutrons. As the universe continued to expand and cool, these particles formed the ...
... is the case because of the Big Bang, when temperatures were so high that only energy could exist. As the universe expanded, it cooled and some of the energy converted into matter in the form of electrons, protons and neutrons. As the universe continued to expand and cool, these particles formed the ...
The Sun`s Size, Heat, and Structure
... largest star known, Epsilon Aurigae, would be the size of a football field. ...
... largest star known, Epsilon Aurigae, would be the size of a football field. ...
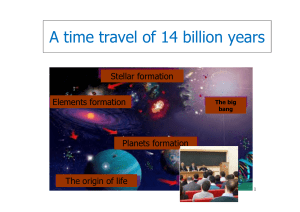
A time travel of 14 billion years
... constituents formed a primordial soup. •Since that moment the Universe expanded and cooled down. Ordered structures were formed: nuclei, atoms, galaxies, ...
... constituents formed a primordial soup. •Since that moment the Universe expanded and cooled down. Ordered structures were formed: nuclei, atoms, galaxies, ...
Making Heavier Metals
... formed in the s-process . The astronomers were able to prove that the Lead cannot originate from the competing "rprocess" that occurs in other environments like supernova explosions. "This is the first detection of a Lead-star", explains Sophie Van Eck from the Institut d'Astronomie et d'Astrophysiq ...
... formed in the s-process . The astronomers were able to prove that the Lead cannot originate from the competing "rprocess" that occurs in other environments like supernova explosions. "This is the first detection of a Lead-star", explains Sophie Van Eck from the Institut d'Astronomie et d'Astrophysiq ...
Nuclear Physics
... An isotope is when atoms of an Element having the same atomic Number but different Neutron and mass number. Hydrogen has 3 isotopes: Hydrogen Deuterium Tritium ...
... An isotope is when atoms of an Element having the same atomic Number but different Neutron and mass number. Hydrogen has 3 isotopes: Hydrogen Deuterium Tritium ...
Futuro da Ci^encia no IAG
... -High mass stars explode as supernovae and produce a GRBs (z ~ 15 ?) GRB at z~8.2 = pop. III? -First generations of low mass stars should be still evolving, identified by a very low metallicity (or no metals) (z ~ 5 to 15) ...
... -High mass stars explode as supernovae and produce a GRBs (z ~ 15 ?) GRB at z~8.2 = pop. III? -First generations of low mass stars should be still evolving, identified by a very low metallicity (or no metals) (z ~ 5 to 15) ...
$doc.title
... “The aim of nuclear astrophysics is to understand those nuclear reacBons that shape much of the nature of the visible universe. Nuclear fusion is the engine of stars; it produces the energy that ...
... “The aim of nuclear astrophysics is to understand those nuclear reacBons that shape much of the nature of the visible universe. Nuclear fusion is the engine of stars; it produces the energy that ...
Loving The Universe
... Substance Of Life Life requires H, C, O, & N All elements of life came from stars that lived AND DIED before the sun formed. Sun is a 2nd or 3rd generation star o 1st stars were pure hydrogen and helium) ...
... Substance Of Life Life requires H, C, O, & N All elements of life came from stars that lived AND DIED before the sun formed. Sun is a 2nd or 3rd generation star o 1st stars were pure hydrogen and helium) ...
Lecture 15 - Deaths of Stars, Supernovae
... Where do the elements in your body come from? • Solar mass star produce elements up to Carbon and Oxygen – these are ejected into planetary nebula and then recycled into new stars and planets • Supernova produce all of the heavier elements – Elements up to Iron can be produced by fusion – Elements ...
... Where do the elements in your body come from? • Solar mass star produce elements up to Carbon and Oxygen – these are ejected into planetary nebula and then recycled into new stars and planets • Supernova produce all of the heavier elements – Elements up to Iron can be produced by fusion – Elements ...
Where Do Chemical Elements Come From?
... force of gravity resulting from all of the matter above the core, and the core collapses under its own weight. ...
... force of gravity resulting from all of the matter above the core, and the core collapses under its own weight. ...
Friday, January 27, 2017 First exam a week from today. Review
... 5 Helium -> Neon (10 protons, 10 neutrons) 6 Helium -> Magnesium (12 protons, 12 neutrons) 7 Helium -> Silicon (14 protons, 14 neutrons) Then Sulfur, Calcium, Titanium. ...
... 5 Helium -> Neon (10 protons, 10 neutrons) 6 Helium -> Magnesium (12 protons, 12 neutrons) 7 Helium -> Silicon (14 protons, 14 neutrons) Then Sulfur, Calcium, Titanium. ...
Nucleosynthesis
Nucleosynthesis is the process that creates new atomic nuclei from pre-existing nucleons, primarily protons and neutrons. The first nuclei were formed about three minutes after the Big Bang, through the process called Big Bang nucleosynthesis. It was then that hydrogen and helium formed to become the content of the first stars, and this primeval process is responsible for the present hydrogen/helium ratio of the cosmos.With the formation of stars, heavier nuclei were created from hydrogen and helium by stellar nucleosynthesis, a process that continues today. Some of these elements, particularly those lighter than iron, continue to be delivered to the interstellar medium when low mass stars eject their outer envelope before they collapse to form white dwarfs. The remains of their ejected mass form the planetary nebulae observable throughout our galaxy.Supernova nucleosynthesis within exploding stars by fusing carbon and oxygen is responsible for the abundances of elements between magnesium (atomic number 12) and nickel (atomic number 28). Supernova nucleosynthesis is also thought to be responsible for the creation of rarer elements heavier than iron and nickel, in the last few seconds of a type II supernova event. The synthesis of these heavier elements absorbs energy (endothermic) as they are created, from the energy produced during the supernova explosion. Some of those elements are created from the absorption of multiple neutrons (the R process) in the period of a few seconds during the explosion. The elements formed in supernovas include the heaviest elements known, such as the long-lived elements uranium and thorium.Cosmic ray spallation, caused when cosmic rays impact the interstellar medium and fragment larger atomic species, is a significant source of the lighter nuclei, particularly 3He, 9Be and 10,11B, that are not created by stellar nucleosynthesis.In addition to the fusion processes responsible for the growing abundances of elements in the universe, a few minor natural processes continue to produce very small numbers of new nuclides on Earth. These nuclides contribute little to their abundances, but may account for the presence of specific new nuclei. These nuclides are produced via radiogenesis (decay) of long-lived, heavy, primordial radionuclides such as uranium and thorium. Cosmic ray bombardment of elements on Earth also contribute to the presence of rare, short-lived atomic species called cosmogenic nuclides.