Changing Matter
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
... and 100.0 g of AgNO3 are mixed together? How many grams of the excess reactant remain ...
Chapter - Imperial Valley College
... • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess at it use lines to represent covalen ...
... • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess at it use lines to represent covalen ...
Jelly Facts - Institute of Food Research
... trapping the water molecules between them. This mixture of water molecules spread evenly in a collagen matrix is known as a hydrocolloid. The concentration of gelatin needs to be about 1% to form jelly The strength of a gelatin-based gel is its bloom strength and can be measured with a penetrometer ...
... trapping the water molecules between them. This mixture of water molecules spread evenly in a collagen matrix is known as a hydrocolloid. The concentration of gelatin needs to be about 1% to form jelly The strength of a gelatin-based gel is its bloom strength and can be measured with a penetrometer ...
What is Double Expeller-Pressed Canola Meal?
... kcal/kg as is). This is higher than other commonly used protein sources for swine, such as; soybean meal, meat meal and distillers grains. ...
... kcal/kg as is). This is higher than other commonly used protein sources for swine, such as; soybean meal, meat meal and distillers grains. ...
Calculations and the Chemical Equation
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
Holt Modern Chemistry Workbook: ch 11
... unit named after the French mathematician and philosopher Blaise Pascal. One pascal, Pa, is equal to the pressure exerted by a force of 1 N acting on an area of 1 m 2 . In many situations, it is more convenient to use the unit kilopascal, kPa. For example, one atmosphere of pressure, 1 atm, is e ...
... unit named after the French mathematician and philosopher Blaise Pascal. One pascal, Pa, is equal to the pressure exerted by a force of 1 N acting on an area of 1 m 2 . In many situations, it is more convenient to use the unit kilopascal, kPa. For example, one atmosphere of pressure, 1 atm, is e ...
Chapter
... • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment – they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess at it – use lines to represent covalen ...
... • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment – they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess at it – use lines to represent covalen ...
View/Open - Minerva Access
... stability or reversibility of a bound-state can be investigated, as previously described for a single environment system (Pokorny and Almeida 2005; Pokorny et al. 2000; Pokorny et al. 2002). The method allows pre-incubation of the peptide with dye-free LUV before adding to the dye-containing LUV. It ...
... stability or reversibility of a bound-state can be investigated, as previously described for a single environment system (Pokorny and Almeida 2005; Pokorny et al. 2000; Pokorny et al. 2002). The method allows pre-incubation of the peptide with dye-free LUV before adding to the dye-containing LUV. It ...
Allosteric Interactions after 50Years
... and one interesting approach was based on the notion of “equivalent monomers.” This idea, formulated in 1965 by Francis Crick and Jeffries Wyman, permitted mathematical simplifications by postulating a hypothetical equilibrium between monomeric units as the basis of allostery. A number of novel equa ...
... and one interesting approach was based on the notion of “equivalent monomers.” This idea, formulated in 1965 by Francis Crick and Jeffries Wyman, permitted mathematical simplifications by postulating a hypothetical equilibrium between monomeric units as the basis of allostery. A number of novel equa ...
Stoichiometery
... A chemical equation is a recipe for making a molecule. This can be written in “shopping list” format: H2 + O2 → H2O But this doesn’t help with specific amounts ...
... A chemical equation is a recipe for making a molecule. This can be written in “shopping list” format: H2 + O2 → H2O But this doesn’t help with specific amounts ...
Chemistry 211
... The Concept of the mole: We talk here about the chemist’s way for counting the amount of “stuff” as compared to some standard. One mole of “stuff” contains the same number of “particles” as there are atoms in one mole of carbon-12 ...
... The Concept of the mole: We talk here about the chemist’s way for counting the amount of “stuff” as compared to some standard. One mole of “stuff” contains the same number of “particles” as there are atoms in one mole of carbon-12 ...
Chapter 3 - Educator
... Once we know the formulas of the reactants and products in a reaction, we can write the unbalanced equation. We then balance the equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation. For most purposes, a balanced equation should contai ...
... Once we know the formulas of the reactants and products in a reaction, we can write the unbalanced equation. We then balance the equation by determining the coefficients that provide equal numbers of each type of atom on each side of the equation. For most purposes, a balanced equation should contai ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... number of molecules as well as the number of moles of each substance ...
... number of molecules as well as the number of moles of each substance ...
Detergents and Surfactants
... The value of detergents in these applications is derived from their amphiphlic nature. Each detergent molecule is characterized by a hydrophilic "head" region and a hydrophobic "tail" region. The result of this characteristic is the formation of thermodynamically stable micelles with hydrophobic cor ...
... The value of detergents in these applications is derived from their amphiphlic nature. Each detergent molecule is characterized by a hydrophilic "head" region and a hydrophobic "tail" region. The result of this characteristic is the formation of thermodynamically stable micelles with hydrophobic cor ...
Document
... – However, the number given for carbon is 12.01, because the carbon found on earth (natural carbon) is a mixture of the isotopes 12C, 13C, and 14C. – All three isotopes have six protons, but they have six, seven, and eight neutrons, respectively. Because natural carbon is a mixture of isotopes, ...
... – However, the number given for carbon is 12.01, because the carbon found on earth (natural carbon) is a mixture of the isotopes 12C, 13C, and 14C. – All three isotopes have six protons, but they have six, seven, and eight neutrons, respectively. Because natural carbon is a mixture of isotopes, ...
Formation of Helical Hairpins during Membrane Protein Integration
... microsomes, but that the introduction of ``turnpromoting'' residues such as Pro, Asn, Arg, or Asp near the middle of the poly-Leu stretch leads to the formation of a helical hairpin.5 As an easily scored marker for the lumenal or cytoplasmic localization of the P2 domain, an N-glycosylation site (As ...
... microsomes, but that the introduction of ``turnpromoting'' residues such as Pro, Asn, Arg, or Asp near the middle of the poly-Leu stretch leads to the formation of a helical hairpin.5 As an easily scored marker for the lumenal or cytoplasmic localization of the P2 domain, an N-glycosylation site (As ...
Document
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
Calculations and the Chemical Equation
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
... Atoms are exceedingly small, yet their masses have been experimentally determined for each of the elements. The periodic table provides atomic masses in atomic mass units (amu). A more practical unit for defining a "collection" of atoms is the mole, Avogadro's number of particles. Calculations based ...
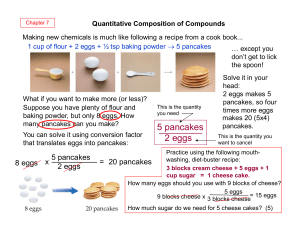
5 pancakes 2 eggs
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
... that is used up during the reaction, i.e. limiting reactant. One bicycle needs 1 frame, 1 seat and 2 wheels, therefore not more than 3 bicycles can be made. The number of seats is the limiting part (reactant); one frame and two wheels are parts in excess; 3 bicycles is the yield. ...
Stoichiometry
... 6. Compound X2Y is 60% X by mass. Calculate the percent Y by mass of the compound XY3? If you have 100 g of X2Y there would be 60g of X and 40g of Y For XY3 since it has only one X atom you can think of that as ½(60g) or 30 g of X and since there’s three Y atoms you can think of that as 3(40g) or 12 ...
... 6. Compound X2Y is 60% X by mass. Calculate the percent Y by mass of the compound XY3? If you have 100 g of X2Y there would be 60g of X and 40g of Y For XY3 since it has only one X atom you can think of that as ½(60g) or 30 g of X and since there’s three Y atoms you can think of that as 3(40g) or 12 ...
Chemistry 1250 - Sp17 Solutions for Midterm 1
... respectively. You have to memorize some polyatomic ions (names, formulas and charges). For molecular compounds the less electronegative element is generally written first in the formula and is named first in the name. The second element (more electronegative element) in the formula is named by using ...
... respectively. You have to memorize some polyatomic ions (names, formulas and charges). For molecular compounds the less electronegative element is generally written first in the formula and is named first in the name. The second element (more electronegative element) in the formula is named by using ...
å¾è湿çå¦
... solution prepared by dissolving 0.10 mol HCOOH in water to make 1.0 L of solution, approximately 4.1% of the HCOOH molecules ionize. What is the pH of this ...
... solution prepared by dissolving 0.10 mol HCOOH in water to make 1.0 L of solution, approximately 4.1% of the HCOOH molecules ionize. What is the pH of this ...
quantitative_chemistry
... In addition to being naturally present in the body, adrenaline is administered as a drug to stimulate the heart, to alleviate allergic reactions, and even to help break up fat cells during liposuction. As you might expect, control over the amounts administered is vital. To make, use, or detect speci ...
... In addition to being naturally present in the body, adrenaline is administered as a drug to stimulate the heart, to alleviate allergic reactions, and even to help break up fat cells during liposuction. As you might expect, control over the amounts administered is vital. To make, use, or detect speci ...
Document
... theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry (using LR only) 1 mol Al 3 mol Cu 0.030 g Al x x = 0.0017 mol Cu 26.98 g Al 2 mol Al • This is different from the actual yield, the amount one actually produces and measures (or experimental) ...
... theoretical yield: the maximum amount of product that can be formed – calculated by stoichiometry (using LR only) 1 mol Al 3 mol Cu 0.030 g Al x x = 0.0017 mol Cu 26.98 g Al 2 mol Al • This is different from the actual yield, the amount one actually produces and measures (or experimental) ...
Empirical and Molecular Formulas
... The mass of C is 48.64g, the mass of H is 8.16g, and the mass of O is 43.20g. Find the Empirical Formula (EF). Step 1: Find Molar mass of each element 48.64 g of C X 1 mol of C/12.01g of C (atomic mass) =4.050 mol of C 8.16 g of H X 1 mol of H/1.008g of H (atomic mass) =8.10 mol of H 43.20 g of O X ...
... The mass of C is 48.64g, the mass of H is 8.16g, and the mass of O is 43.20g. Find the Empirical Formula (EF). Step 1: Find Molar mass of each element 48.64 g of C X 1 mol of C/12.01g of C (atomic mass) =4.050 mol of C 8.16 g of H X 1 mol of H/1.008g of H (atomic mass) =8.10 mol of H 43.20 g of O X ...