Chapter 3: Calculations with Chemical Formulas
... The compound contains 68.8% C, 5.0% H and 26.2% O by mass. What is the empirical formula? For a 100.0-g sample of benzoic acid, 68.8 g are C, 5.0 g are H, and 26.2 g are O. Using the molar masses, convert these masses to moles: 68.8g C x 1 mol C = 5.729 mol C 12.01g C 5.0g H x 1 mol H = 4.961 mol H ...
... The compound contains 68.8% C, 5.0% H and 26.2% O by mass. What is the empirical formula? For a 100.0-g sample of benzoic acid, 68.8 g are C, 5.0 g are H, and 26.2 g are O. Using the molar masses, convert these masses to moles: 68.8g C x 1 mol C = 5.729 mol C 12.01g C 5.0g H x 1 mol H = 4.961 mol H ...
Practice Exercise 1
... Begin by counting each kind of atom on the two sides of the arrow. There are one Na, one O, and two H on the left side, and one Na, one O, and three H on the right. The Na and O atoms are balanced, but the number of H atoms is not. To increase the number of H atoms on the left, let’s try placing the ...
... Begin by counting each kind of atom on the two sides of the arrow. There are one Na, one O, and two H on the left side, and one Na, one O, and three H on the right. The Na and O atoms are balanced, but the number of H atoms is not. To increase the number of H atoms on the left, let’s try placing the ...
03_Worked_Examples
... Begin by counting each kind of atom on the two sides of the arrow. There are one Na, one O, and two H on the left side, and one Na, one O, and three H on the right. The Na and O atoms are balanced, but the number of H atoms is not. To increase the number of H atoms on the left, let’s try placing the ...
... Begin by counting each kind of atom on the two sides of the arrow. There are one Na, one O, and two H on the left side, and one Na, one O, and three H on the right. The Na and O atoms are balanced, but the number of H atoms is not. To increase the number of H atoms on the left, let’s try placing the ...
Transmembrane Fragment Structures of Amyloid Precursor Protein
... our simulations, G29-L52 distances in the range of 33−34 Å are inconsistent with the presence of a structural kink in the TM helix near G37/G38. Consistent with this view, the G29-L52 distances derived from the PDB structures (orange lines in Figure 5) lead to a significantly shorter average distance ...
... our simulations, G29-L52 distances in the range of 33−34 Å are inconsistent with the presence of a structural kink in the TM helix near G37/G38. Consistent with this view, the G29-L52 distances derived from the PDB structures (orange lines in Figure 5) lead to a significantly shorter average distance ...
Ch 3
... A fuel mixture used in the early days of rocketry is composed of two liquids, hydrazine(N2H4) and dinitrogen tetraoxide(N2O4), which ignite on contact to form nitrogen gas and water vapor. How many grams of nitrogen gas form when 1.00x102g of N2H4 and 2.00x102g of N2O4 are mixed? ...
... A fuel mixture used in the early days of rocketry is composed of two liquids, hydrazine(N2H4) and dinitrogen tetraoxide(N2O4), which ignite on contact to form nitrogen gas and water vapor. How many grams of nitrogen gas form when 1.00x102g of N2H4 and 2.00x102g of N2O4 are mixed? ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... One mole of atoms, ions, or molecules contains Avogadro’s number of those particles One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
CHAPTER 20 METALLURGY AND THE CHEMISTRY OF METALS
... than that of water. The tetrachloride can be formed by treating the oxide (rutile) with chlorine gas at high temperature. The balanced equation is: TiO2(s) + 2Cl2(g) → TiCl4(l) + O2(g) The liquid tetrachloride can be isolated and purified by simple distillation. Purified TiCl4 is then reduced with m ...
... than that of water. The tetrachloride can be formed by treating the oxide (rutile) with chlorine gas at high temperature. The balanced equation is: TiO2(s) + 2Cl2(g) → TiCl4(l) + O2(g) The liquid tetrachloride can be isolated and purified by simple distillation. Purified TiCl4 is then reduced with m ...
Chapter 2 - Chemistry
... Chapter goals • Understand the nature of ionic substances dissolved in water. • Recognize common acids and bases and understand their behavior in aqueous solution. • Recognize and write equations for the common types of reactions in aqueous solution. • Recognize common oxidizing and reducing agents ...
... Chapter goals • Understand the nature of ionic substances dissolved in water. • Recognize common acids and bases and understand their behavior in aqueous solution. • Recognize and write equations for the common types of reactions in aqueous solution. • Recognize common oxidizing and reducing agents ...
Stoichiometry and the Mole
... If any of the ratios are not a whole number, multiply all the ratios by a factor to make it a whole number – If ratio is ?.5 then multiply by 2; if ?.33 or ?.67 then multiply by 3; if ?.25 or ?.75 then multiply by 4 ...
... If any of the ratios are not a whole number, multiply all the ratios by a factor to make it a whole number – If ratio is ?.5 then multiply by 2; if ?.33 or ?.67 then multiply by 3; if ?.25 or ?.75 then multiply by 4 ...
Appendices and Glossary
... label approach for nearly all of the stoichiometric calculations in this book. A.3 MOLAR MASSES AND ATOMIC WEIGHTS OF THE ELEMENTS The number under the symbol of an element on the periodic table is the element’s atomic weight. It represents the “average atomic weight” or “average atomic mass” of the ...
... label approach for nearly all of the stoichiometric calculations in this book. A.3 MOLAR MASSES AND ATOMIC WEIGHTS OF THE ELEMENTS The number under the symbol of an element on the periodic table is the element’s atomic weight. It represents the “average atomic weight” or “average atomic mass” of the ...
Combining docking and molecular dynamic simulations in drug design
... to slow conformational changes or even protein folding processes.48 It is also possible to study the effect of explicit solvent molecules on protein structure and stability to obtain time-averaged properties of the biomolecular system, such as density, conductivity, and dipolar moment, as well as di ...
... to slow conformational changes or even protein folding processes.48 It is also possible to study the effect of explicit solvent molecules on protein structure and stability to obtain time-averaged properties of the biomolecular system, such as density, conductivity, and dipolar moment, as well as di ...
Official Drugstore. Can You Take Cialis With Lisinopril
... If 22.4 g of hydrogen and 177.6 g of oxygen are formed, how many grams of water reacted? Conservation of mass mass of reactants = mass of products mass of H2O = mass of H2 + mass of O2 mass of H2O = 22.4 g + 177.6 g mass of H2O = 200.0 g ...
... If 22.4 g of hydrogen and 177.6 g of oxygen are formed, how many grams of water reacted? Conservation of mass mass of reactants = mass of products mass of H2O = mass of H2 + mass of O2 mass of H2O = 22.4 g + 177.6 g mass of H2O = 200.0 g ...
Stoichiometry Objectives
... To convert between moles and mass, you need to use the atomic mass found on the periodic table. Calculate the mass of 0.625 moles of calcium. -According to the periodic table, the atomic mass of calcium is 40.078 amu, so the molar mass of calcium is 40.078 g/mol. ...
... To convert between moles and mass, you need to use the atomic mass found on the periodic table. Calculate the mass of 0.625 moles of calcium. -According to the periodic table, the atomic mass of calcium is 40.078 amu, so the molar mass of calcium is 40.078 g/mol. ...
Gases - chemmybear.com
... It has the largest size or volume. It has the strongest attractive forces (van der Waals forces or dipole-dipole interactions). (c) High temperature result in high kinetic energies. This energy overcomes the attractive forces. Low pressure increases the distance between molecules. (So molecules comp ...
... It has the largest size or volume. It has the strongest attractive forces (van der Waals forces or dipole-dipole interactions). (c) High temperature result in high kinetic energies. This energy overcomes the attractive forces. Low pressure increases the distance between molecules. (So molecules comp ...
Unit 6- Math of Chemistry
... • What do we know about moles? – # of atoms in 12 grams of C-12 – Avogadro’s number – 6.022 x 1023 ...
... • What do we know about moles? – # of atoms in 12 grams of C-12 – Avogadro’s number – 6.022 x 1023 ...
Chapter 3 Mass Relationships in Chemical Reactions 1
... (carbon dioxide and water) is 93%. What mass of carbon dioxide will be produced in this engine when 15.0 g of octane is burned with 15.0 g of oxygen gas? (b) 12. g3 (c) 21 g (d) 54. g (a) 13. g 105. The Hall process for the production of aluminum involves the reaction of aluminum oxide with elementa ...
... (carbon dioxide and water) is 93%. What mass of carbon dioxide will be produced in this engine when 15.0 g of octane is burned with 15.0 g of oxygen gas? (b) 12. g3 (c) 21 g (d) 54. g (a) 13. g 105. The Hall process for the production of aluminum involves the reaction of aluminum oxide with elementa ...
Electrophoresis
... Sodium dodecylsulfate (SDS) is an anionic detergent which denatures secondary and non–disulfide–linked tertiary structures by wrapping around the polypeptide backbone. In so doing, SDS confers a net negative charge to the polypeptide in proportion to its length When treated with SDS and a reducing a ...
... Sodium dodecylsulfate (SDS) is an anionic detergent which denatures secondary and non–disulfide–linked tertiary structures by wrapping around the polypeptide backbone. In so doing, SDS confers a net negative charge to the polypeptide in proportion to its length When treated with SDS and a reducing a ...
Calculations from Balanced Equations
... Excess reactants You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reacti ...
... Excess reactants You can use the relative numbers of moles of substances, as shown in balanced equations, to calculate the amounts of reactants needed or the amounts of products produced. A limiting reactant is the substance that is fully used up and thereby limits the possible extent of the reacti ...
Heriot-Watt University Scattering Dynamics of Oxygen Atoms on
... is to transform a Maxwell-Boltzmann distribution of translational energies at the surface temperature to a TOF distribution and adjust its amplitude to fit the slow (TD) component (red curves in Fig. 2). Then the IS component of the TOF is determined by subtracting the fitted TD component from the ...
... is to transform a Maxwell-Boltzmann distribution of translational energies at the surface temperature to a TOF distribution and adjust its amplitude to fit the slow (TD) component (red curves in Fig. 2). Then the IS component of the TOF is determined by subtracting the fitted TD component from the ...
unit iv – stoichiometry 1
... * Chemical Formulas - a series of symbols and numbers used to represent the composition of an element or a compound I. Formulas for Compounds A. Ionic Compounds - represents ratio of cations to anions * remember, ionic compounds don’t form molecules as we think of them * we call “molecules” formula ...
... * Chemical Formulas - a series of symbols and numbers used to represent the composition of an element or a compound I. Formulas for Compounds A. Ionic Compounds - represents ratio of cations to anions * remember, ionic compounds don’t form molecules as we think of them * we call “molecules” formula ...
Molecules, Moles and Chemical Equations File
... oday, roughly 20 million chemical compounds are known. More are being discovered or created as you read this. Although chemical nomenclature provides a systematic way to facilitate discussion of this tremendous diversity of substances, that is just a small step toward understanding chemistry. Much o ...
... oday, roughly 20 million chemical compounds are known. More are being discovered or created as you read this. Although chemical nomenclature provides a systematic way to facilitate discussion of this tremendous diversity of substances, that is just a small step toward understanding chemistry. Much o ...
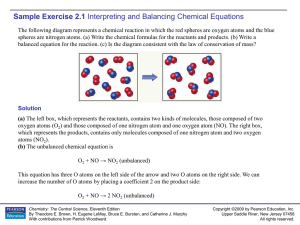
Sample Exercise 2.1
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
... Write the balanced equation for the reaction that occurs when methanol, CH 3OH(l), is burned in air. Solution When any compound containing C, H, and O is combusted, it reacts with the O 2(g) in air to produce CO2(g) and H2O(g). Thus, the unbalanced equation is CH3OH(l) + O2(g) → CO2(g) + H2O(g) In t ...
a) How many moles of water are created when 108 moles of oxygen
... work as is explained in the lessons. You are required to have this package completed BEFORE you write your unit test. Do your best and ask questions if you don’t understand anything! ...
... work as is explained in the lessons. You are required to have this package completed BEFORE you write your unit test. Do your best and ask questions if you don’t understand anything! ...