chapter 5 gases
... (a) is a strong electrolyte. The compound dissociates completely into ions in solution. (b) is a nonelectrolyte. The compound dissolves in water, but the molecules remain intact. (c) is a weak electrolyte. A small amount of the compound dissociates into ions in water. When NaCl dissolves in water it ...
... (a) is a strong electrolyte. The compound dissociates completely into ions in solution. (b) is a nonelectrolyte. The compound dissolves in water, but the molecules remain intact. (c) is a weak electrolyte. A small amount of the compound dissociates into ions in water. When NaCl dissolves in water it ...
CHAPTER 4 REACTIONS IN AQUEOUS SOLUTIONS
... solution. An ionic equation will show strong acids and strong bases in terms of their free ions. Weak acids and weak bases are weak electrolytes. They only ionize to a small extent in solution. Weak acids and weak bases are shown as molecules in ionic and net ionic equations. A net ionic equation sh ...
... solution. An ionic equation will show strong acids and strong bases in terms of their free ions. Weak acids and weak bases are weak electrolytes. They only ionize to a small extent in solution. Weak acids and weak bases are shown as molecules in ionic and net ionic equations. A net ionic equation sh ...
CHAPTER 3 STOICHIOMETRY
... Solution: Let's first calculate the number of N atoms in 1.68 10 g of urea. First, we must convert grams of urea to number of molecules of urea. This calculation is similar to Problem 3.26. The molecular formula of urea shows there are two N atoms in one urea molecule, which will allow us to conve ...
... Solution: Let's first calculate the number of N atoms in 1.68 10 g of urea. First, we must convert grams of urea to number of molecules of urea. This calculation is similar to Problem 3.26. The molecular formula of urea shows there are two N atoms in one urea molecule, which will allow us to conve ...
4. chemical reactions
... Note on significant figures: If the final answer to a solution needs to be rounded off, it is given first with one nonsignificant figure, and the last significant figure is underlined. The final answer is then rounded to the correct number of significant figures. In multiple-step problems, intermedi ...
... Note on significant figures: If the final answer to a solution needs to be rounded off, it is given first with one nonsignificant figure, and the last significant figure is underlined. The final answer is then rounded to the correct number of significant figures. In multiple-step problems, intermedi ...
Announcements - University of Illinois Urbana
... • Magnitude of G(T) to R(T) curve determines if reactor T will rise or fall • G(T) = R(T) intersection, equal rate of heat generation & removal, no change in T • G(T) > R(T) (G(T) line above R(T) on graph): rate of heat generation > heat removal, so reactor heats up until a steady state is reached • ...
... • Magnitude of G(T) to R(T) curve determines if reactor T will rise or fall • G(T) = R(T) intersection, equal rate of heat generation & removal, no change in T • G(T) > R(T) (G(T) line above R(T) on graph): rate of heat generation > heat removal, so reactor heats up until a steady state is reached • ...
Chapter 8 PowerPoint - Southeast Online
... • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecules NH3 • Since we count molecules by moles: 3 moles H2 1 mole N2 2 moles NH3 Tro's “Introductory Chemist ...
... • The equation 3 H2(g) + N2(g) 2 NH3(g) tells us that 3 molecules of H2 react with exactly 1 molecule of N2 and make exactly 2 molecules of NH3 or: 3 molecules H2 1 molecule N2 2 molecules NH3 • Since we count molecules by moles: 3 moles H2 1 mole N2 2 moles NH3 Tro's “Introductory Chemist ...
Stoichiometry of Formulas and Equations
... the left (reactants) as on the right ( products) but in different combinations; we can therefore use the amount of one substance to calculate the amount of any other. ◆ During a typical reaction, one substance (the limiting reactant) is used up, so it limits the amount of product that can form; the ...
... the left (reactants) as on the right ( products) but in different combinations; we can therefore use the amount of one substance to calculate the amount of any other. ◆ During a typical reaction, one substance (the limiting reactant) is used up, so it limits the amount of product that can form; the ...
Chapter 4
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
Stoichiometry - Normal Community High School Chemistry
... reach from the Sun to Pluto and back 7.5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills. ...
... reach from the Sun to Pluto and back 7.5 million times. It would take light 9500 years to travel from the bottom to the top of a stack of 1 mole of $1 bills. ...
Stoichiometric Calculations
... that will determine the maximum amount of product you can get, also called the maximum yield. There are a variety of methods to determine which reactant is the limiting one. ...
... that will determine the maximum amount of product you can get, also called the maximum yield. There are a variety of methods to determine which reactant is the limiting one. ...
Stoichiometric Calculations
... Limiting reagent problems are worded differently because the quantities of both reactants are given. 10 moles of H2 and 20 moles of Cl2 react to produce HCl. Which quantity is the limiting reagent? It is your job to figure out which reactant is limiting because that will determine the maximum amount ...
... Limiting reagent problems are worded differently because the quantities of both reactants are given. 10 moles of H2 and 20 moles of Cl2 react to produce HCl. Which quantity is the limiting reagent? It is your job to figure out which reactant is limiting because that will determine the maximum amount ...
Chapter 12
... the reactants. We have to calculate how much of a product we can get from each of the reactants to determine which reactant is the limiting one. The lower amount of a product is the correct ...
... the reactants. We have to calculate how much of a product we can get from each of the reactants to determine which reactant is the limiting one. The lower amount of a product is the correct ...
Moles 1 - pedagogics.ca
... and stoichiometry is the study of the ratios in which chemical substances combine. In order to know the exact quantity of each substance that is required to react we need to know the number of atoms, molecules or ions present in a specific amount of that substance. However, the mass of an individual ...
... and stoichiometry is the study of the ratios in which chemical substances combine. In order to know the exact quantity of each substance that is required to react we need to know the number of atoms, molecules or ions present in a specific amount of that substance. However, the mass of an individual ...
Answers to Problem-Solving Practice Problems
... (c) Heterogeneous mixture of dirt and oil (d) Element; diamond is pure carbon. (e) Modern quarters (since 1965) are composed of a pure copper core (that can be seen when they are viewed side-on) and an outer layer of 75% Cu, 25% Ni alloy, so they are heterogeneous matter. Pre-1965 quarters are fairl ...
... (c) Heterogeneous mixture of dirt and oil (d) Element; diamond is pure carbon. (e) Modern quarters (since 1965) are composed of a pure copper core (that can be seen when they are viewed side-on) and an outer layer of 75% Cu, 25% Ni alloy, so they are heterogeneous matter. Pre-1965 quarters are fairl ...
Noncovalent interactions of molecules with single walled carbon
... assumed to be uniform over the surface of the nanotube. The continuum and discrete approaches give similar values for energy of interaction, usually within a few percent for most systems.34,37 If the effect of NT sidewall corrugation is of interest, then the discrete approach is necessary, since a c ...
... assumed to be uniform over the surface of the nanotube. The continuum and discrete approaches give similar values for energy of interaction, usually within a few percent for most systems.34,37 If the effect of NT sidewall corrugation is of interest, then the discrete approach is necessary, since a c ...
Stoichiometric Calculations
... Stoichiometry is the quantitative determination of reactants or products using a balanced chemical equation. We can interpret a balanced equation in several ways. The most common way is to interpret it as indicating the number of moles of each substance. For instance, this equation, 3 H2 + N2 ® 2 NH ...
... Stoichiometry is the quantitative determination of reactants or products using a balanced chemical equation. We can interpret a balanced equation in several ways. The most common way is to interpret it as indicating the number of moles of each substance. For instance, this equation, 3 H2 + N2 ® 2 NH ...
Stoichiometry - Social Circle City Schools
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
... As you learned in Unit 1, atoms are so small and have such small masses that any amount of atoms we would work with would be very hard to count. For example, a piece of aluminum about the size of a pencil eraser contains approximately 2 × 1022 aluminum atoms! The mole (abbreviated mol) is the unit c ...
Topic 1 Quantitative Chemistry Answers - slider-dpchemistry-11
... Ratio = 1:2, therefore the empirical formula = MgO2 b) The results may not match with the actual empirical formula and even if they are correct, we make assumptions about the experiment in calculating the results. List four assumptions made. The sample of magnesium is pure. All of the magnesium is b ...
... Ratio = 1:2, therefore the empirical formula = MgO2 b) The results may not match with the actual empirical formula and even if they are correct, we make assumptions about the experiment in calculating the results. List four assumptions made. The sample of magnesium is pure. All of the magnesium is b ...
SCH3U: Final Exam Review Note: These questions a
... 48. Find the concentration of the following compounds a) pH = 0.1 of HCl b) pH = 2.4 of HCH3CO2 c) pH = 0.014 H2SO4 49. Identify the conjugate acid-base pairs in the following reaction: H3PO4 (aq) + H2O(l) → H2PO4-(aq) + H3O+(aq) 50. Name each acid. a) HBr(aq) b) H3PO2(aq) c) H2SO3(aq) d) HIO3(aq) e ...
... 48. Find the concentration of the following compounds a) pH = 0.1 of HCl b) pH = 2.4 of HCH3CO2 c) pH = 0.014 H2SO4 49. Identify the conjugate acid-base pairs in the following reaction: H3PO4 (aq) + H2O(l) → H2PO4-(aq) + H3O+(aq) 50. Name each acid. a) HBr(aq) b) H3PO2(aq) c) H2SO3(aq) d) HIO3(aq) e ...
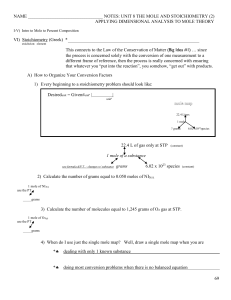
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... There are many types of homogeneous solutions. The "aqueous solution" is just one type. It is however, the most important to the understanding of the material contained in this Chemistry syllabus. ...
... There are many types of homogeneous solutions. The "aqueous solution" is just one type. It is however, the most important to the understanding of the material contained in this Chemistry syllabus. ...
Chapter+12
... What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
... What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
Cookies and Chemistry…Huh!?!?
... What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
... What if we had 3 moles of oxygen, how much hydrogen would we need to react and how much water would we get? What if we had 50 moles of hydrogen, how much oxygen would we need and how much water produced? ...
Chemistry 11 Final Examination Review
... 9. Which of the following ideas of the Bohr model is not retained in the modern theory of ...
... 9. Which of the following ideas of the Bohr model is not retained in the modern theory of ...
8. Solution Guide to Supplementary Exercises
... = 0.0880 mol According to the equation of Reaction 2, 1 mole of HCO3–(aq) would react with 1 mole of H+(aq). i.e. 0.0800 mole of HCO3–(aq) would react with 0.0800 mole of H+(aq). Hence H+(aq) was in excess. HCO3–(aq) was the limiting reactant. ...
... = 0.0880 mol According to the equation of Reaction 2, 1 mole of HCO3–(aq) would react with 1 mole of H+(aq). i.e. 0.0800 mole of HCO3–(aq) would react with 0.0800 mole of H+(aq). Hence H+(aq) was in excess. HCO3–(aq) was the limiting reactant. ...
Chapter 3 Stoichiometry
... is called Avogadro’s number (NA) and has a value of 6.0221415 × 1023. In most cases we will use 6.022 × 1023 or 6.02 × 1023 for Avogadro’s number. One mole of any element contains 6.0221415 × 1023 atoms of that element, and one mole of a molecular compound contains 6.0221415 × 1023 molecules of t ...
... is called Avogadro’s number (NA) and has a value of 6.0221415 × 1023. In most cases we will use 6.022 × 1023 or 6.02 × 1023 for Avogadro’s number. One mole of any element contains 6.0221415 × 1023 atoms of that element, and one mole of a molecular compound contains 6.0221415 × 1023 molecules of t ...